

Lintuzumab Overview
Introduction of Lintuzumab
Lintuzumab (SGN-33) is a humanized monoclonal antibody used in the treatment of cancer. The drug had been developed by Seattle Genetics as a treatment for acute myeloid leukemia (AML). Lintuzumab targets the CD33 protein, which is expressed in AML and other myeloproliferative diseases, but does not appear in abundance on normal cells. Trials for AML were abandoned in 2010 when a phase IIb trial failed to show increased survival. As of 2010, Seattle Genetics was conducting Phase II trials of lintuzumab in conjunction with bortezomib (marketed as Velcade) as a treatment for those with myelodysplastic syndromes. Lintuzumab had been in mid-stage clinical trial when Seattle Genetics pulled the drug in September 2010 after evidence showed that it did not lead to higher survival rates. The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) had granted lintuzumab orphan drug status for treatment of AML and myelodysplastic syndromes. Seattle Genetics had licensed lintuzumab from PDL BioPharma, which had been unsuccessful in treating AML in clinical trials of its own in which they used lower doses. The Phase IIb randomized, double-blind clinical trial studied 211 individuals ages 60 and over who had been enrolled by February 2009 and who were poor candidates for high-dose chemotherapy or had made the choice to reject the traditional chemotherapy treatment. The study participants typically had a projected four to five months to live, with half treated with lintuzumab and a low dose of the chemotherapeutic agent cytarabine, while the other half were given cytarabine in combination with a placebo. No patients were harmed in the trial and patients in both groups lived longer than expected, with those being given lintuzumab having a lower death rate. However, the study found that there was no benefit to patients on a statistical basis, and that it did not reduce the risk of infection or the need for blood transfusions.
Mechanism of Action of Lintuzumab
CD33 is a member of the sialic acid binding immunoglobulin(Ig) like lectins (Siglecs), a subset of the immunoglobulin superfamily molecules. CD33 is an attractive therapeutic target in AML due to the frequent expression of this transmembrane glycoprotein on adult and childhood AML blasts and importantly identified on adult LSCs. Extracellularly, CD33 has two Ig-like domains: V set Ig like domain and C2 set Ig-like domain. Intracellularly, the cytoplasmic tail has one immunoreceptor tyrosine based inhibitory motif (ITIM) and a second ITIM-like tyrosine residue. CD33 has endocytic properties when bound by bivalent antibodies that result in internalization of the antigen/antibody complex. Therefore, binding of an antibody reduces the cell surface expression of CD33 but new CD33 sites are continuously expressed. Intracellular mutations in the ITIMs have also been shown to impair the endocytic properties of the receptor and subsequent internalization of the antibody-CD33 complex. The function of CD33 in normal and AML cells remains largely unknown. There is increasing evidence that it negatively regulates inflammatory and immune responses through inhibitory effects on tyrosine kinase-driven signalling pathways. Lintuzumab targets CD33 and has shown therapeutic efficacy against AML.
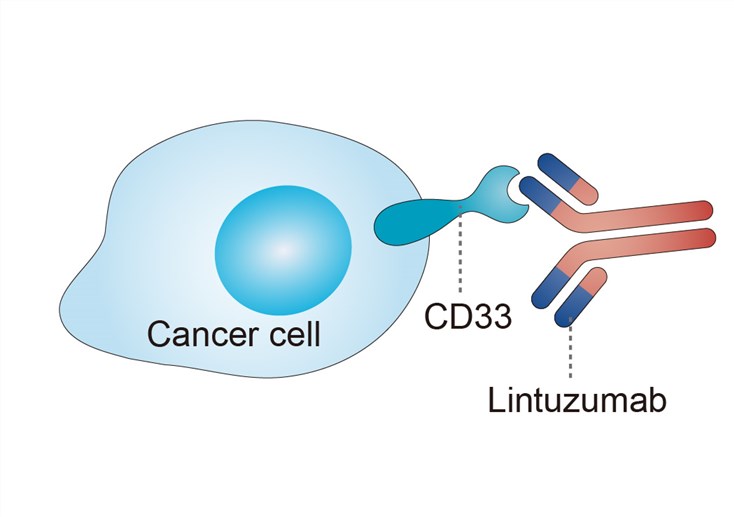 Fig.1 Mechanism of action of lintuzumab
Fig.1 Mechanism of action of lintuzumab
Table 1. Clinical Projects of Lintuzumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03441048 | Recruiting | Acute Myeloid Leukemia | Medical College of Wisconsin | February 21, 2018 |
| NCT03705858 | Not yet recruiting | Acute Myeloid Leukemia | Joseph Jurcic | October 15, 2018 |
| NCT02575963 | Recruiting | AML | Actinium Pharmaceuticals | October 15, 2015 |
| NCT02998047 | Recruiting | Refractory Multiple Myeloma | Actinium Pharmaceuticals | December 20, 2016 |
What We Provide
Therapeutic Antibody
Lintuzumab
We provide high-quality Lintuzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, notfor diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Lintuzumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

