

Mepolizumab Overview
Introduction of Mepolizumab
Mepolizumab (SB-240563) is a high-affnity, humanized monoclonal antibody of the IgG1/κ subtype, specifc for Interleukin-5(IL-5). Mepolizumab is indicated for add-on maintenance treatment of severe eosinophilic asthma in patients aged 12 years and older. It was approved by the FDA in November, 2015 for the treatment of asthma under the brand name Nucala (marketed by GlaxoSmithKline). Additional, mepolizumab has been investigated in the treatment of severe nasal polyposis, among numerous other conditions.
Mechanism of Action of Mepolizumab
As eosinophils are the dominating effector cells in asthma, inhibition of eosinophilia, theoretically would result in a decreased airway injury, mucus hypersecretion and bronchial hyper-responsiveness. IL-5 induces an inflammatory response by interacting with eosinophils through targeting the Interleukin-5 receptor (IL-5R) expressed in eosinophils, basophils and some mast cells. Mepolizumab is a fully humanized mAb specific for human IL-5, which blocks binding of human IL-5 to the α chain of the IL-5R complex, thus inhibits bioactivity of IL-5. Blocking of IL-5 signalling thereby reduces the production and survival of eosinophils and inhibits eosinophilic-driven inflammation.
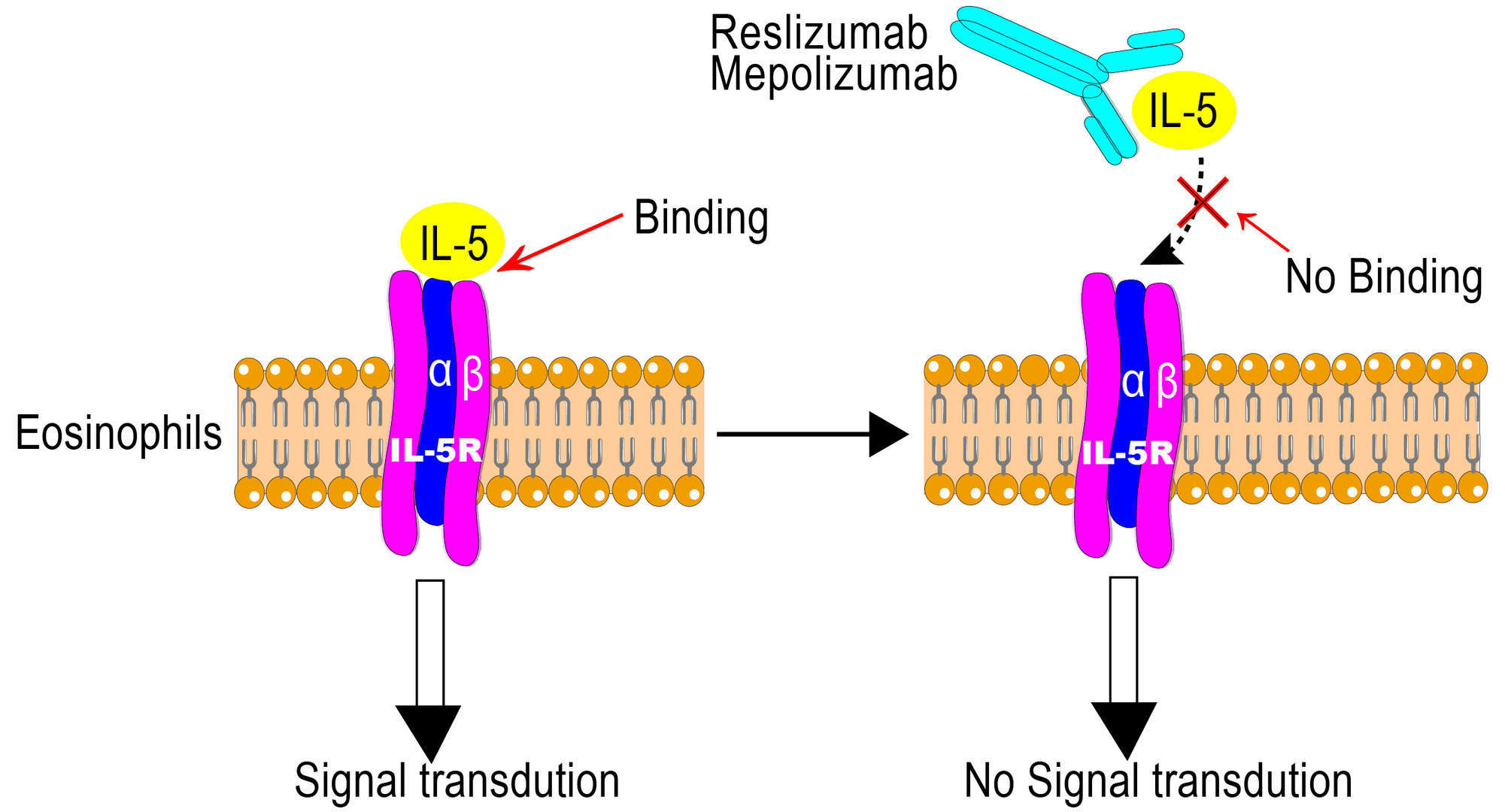 Figure 1 Mechanism of Action of Mepolizumab
Figure 1 Mechanism of Action of Mepolizumab
Clinical Projects of Mepolizumab *
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03306043 | Recruiting | Hypereosinophilic Syndrome | GlaxoSmithKline | June 15, 2018 |
| NCT00802438 | Active, not recruiting | Asthma | University of Wisconsin, Madison | December 12, 2017 |
| NCT03476109 | Not yet recruiting | Severe Asthma | Cliniques universitaires Saint-Luc- Université Catholique de Louvain | March 27, 2018 |
| NCT02555371 | Recruiting | Asthma | GlaxoSmithKline | January 17, 2018 |
| NCT03494881 | Not yet recruiting | Chronic Spontaneous Urticaria | Mayo Clinic | April 11, 2018 |
| NCT03292588 | Recruiting | Asthma | National Institute of Allergy and Infectious Diseases (NIAID) | November 6, 2017 |
| NCT03298061 | Active, not recruiting | Churg-Strauss Syndrome | GlaxoSmithKline | June 1, 2018 |
| NCT03085797 | Recruiting | Polyps, Nasal | GlaxoSmithKline | June 12, 2018 |
| NCT02836496 | Recruiting | Hypereosinophilic Syndrome | GlaxoSmithKline | June 12, 2018 |
| NCT03557060 | Not yet recruiting | Churg-Strauss Syndrome | GlaxoSmithKline | June 14, 2018 |
| NCT03028480 | Active, not recruiting | Asthma | GlaxoSmithKline | February 6, 2018 |
| NCT00244686 | Available | Hypereosinophilic Syndrome | GlaxoSmithKline | April 13, 2018 |
| NCT03324230 | Recruiting | Asthma Brittle | Queen's University, Belfast | April 13, 2018 |
| NCT03470311 | Not yet recruiting | Severe Prednisone Dependent Eosinophilic Asthma | McMaster University | March 19, 2018 |
| NCT03436511 | Recruiting | Pulmonary Disease, Chronic Obstructive | GlaxoSmithKline | February 28, 2018 |
| NCT03137043 | Recruiting | Asthma | GlaxoSmithKline | March 22, 2018 |
Approved Drugs of Mepolizumab **
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| NUCALA | Refractory eosinophilic asthma | Injection, powder for | 100mg | Subcutaneous | GlaxoSmithKline Australia Pty Ltd | February 2, 2016 |

|
| NUCALA | Severe asthma | Injection, powder for | 100 mg/mL | Subcutaneous | GlaxoSmithKlineLnc | October 14, 2015 |

|
| NUCALA | Refractory eosinophilic asthma | Injection, powder for | 100mg | Subcutaneous | GlaxoSmithKlineLnc | March 14, 2016 |

|
What We Provide
Therapeutic Antibody
Mepolizumab
We provide high-quality Mepolizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Mepolizumab&cntry=&state=&city=&dist=
** Information presented in the table were collected from the following websites:
http://search.tga.gov.au/s/search.html?collection=tgaartg&profile=record&meta_i=232028
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/125526Orig1s000SumR.pdf
https://www.drugbank.ca/drugs/DB06612
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

