

Nimotuzumab Overview
Introduction of Nimotuzumab
Nimotuzumab is a humanized IgG1 monoclonal antibody (mAb) that as of 2014 had orphan status in the U.S. and European Union for glioma, and marketing approval in India, China, and other countries for squamous cell carcinomas of the head and neck (SCCHN), and was undergoing several clinical trials. It was developed at the Center of molecular immunology (CIM) in Havana, Cuba. Nimotuzumab binds to the epidermal growth factor receptor (EGFR), a signalling protein that normally controls cell division. Nimotuzumab was obtained by transplanting the complementarity determining regions (CDRs) of the murine IgG2a monoclonal ior egf/r3, to a human framework assisted by computer modeling. The parental murine monoclonal ior egf/r3 was generated by fusing the murine myeloma cells SP2/Ag14 with splenocytes from BALB/c mice immunized with a purified human placenta fraction enriched in EGFR and not with EGFR purified from cultured cells.
Mechanism of Action of Nimotuzumab
EGFR, also known as erb B1 or HER1, is a receptor tyrosine kinase (RTK) belonging, along with erb B2 (or HER 2), erb B3 and erb B4, to the RTK family of EGFR. When EGFR is bound to its ligand, dimerization occurs (homodimerizes with another EGFR or heterodimerizes with a different receptor of the same family) and a signaling cascade begins at intracellular level, activating, among others, the MAPK (mitogen-activated protein kinase), the STAT (signal transducer and activator of transcription) and the Akt antiapoptotic kinase pathways, different genes being eventually activated, and thus cellular response being produced. EGFR transmitted signal is inactivated by receptor internalization, and its degradation or recycling. EGFR is expressed in healthy tissue and in many tumors, particularly in those of epithelial origin, and its activation plays a significant role in tumorigenesis, by stimulating cell proliferation and inhibiting apoptosis. It also favors angiogenesis and facilitates metastasis generation. EGFR expression increase has been observed in many tumors. This EGFR overexpression usually correlates with a more advanced stage of the disease, a poorer prognosis and a worst response to chemotherapy. In preclinical models it was also found that the inhibition of these receptors had anti-tumour activity, and data available suggested synergy with chemotherapy as well as radiotherapy. Nimotuzumab binds with optimal affinity and high specificity to the extracellular region of EGFR, resulting in a blockade of ligand binding and receptor activation. In addition, as a IgG1 mAb, nimotuzumab could also be cytolytic on target tumors by its capacity to cause antibody dependent cell mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC).
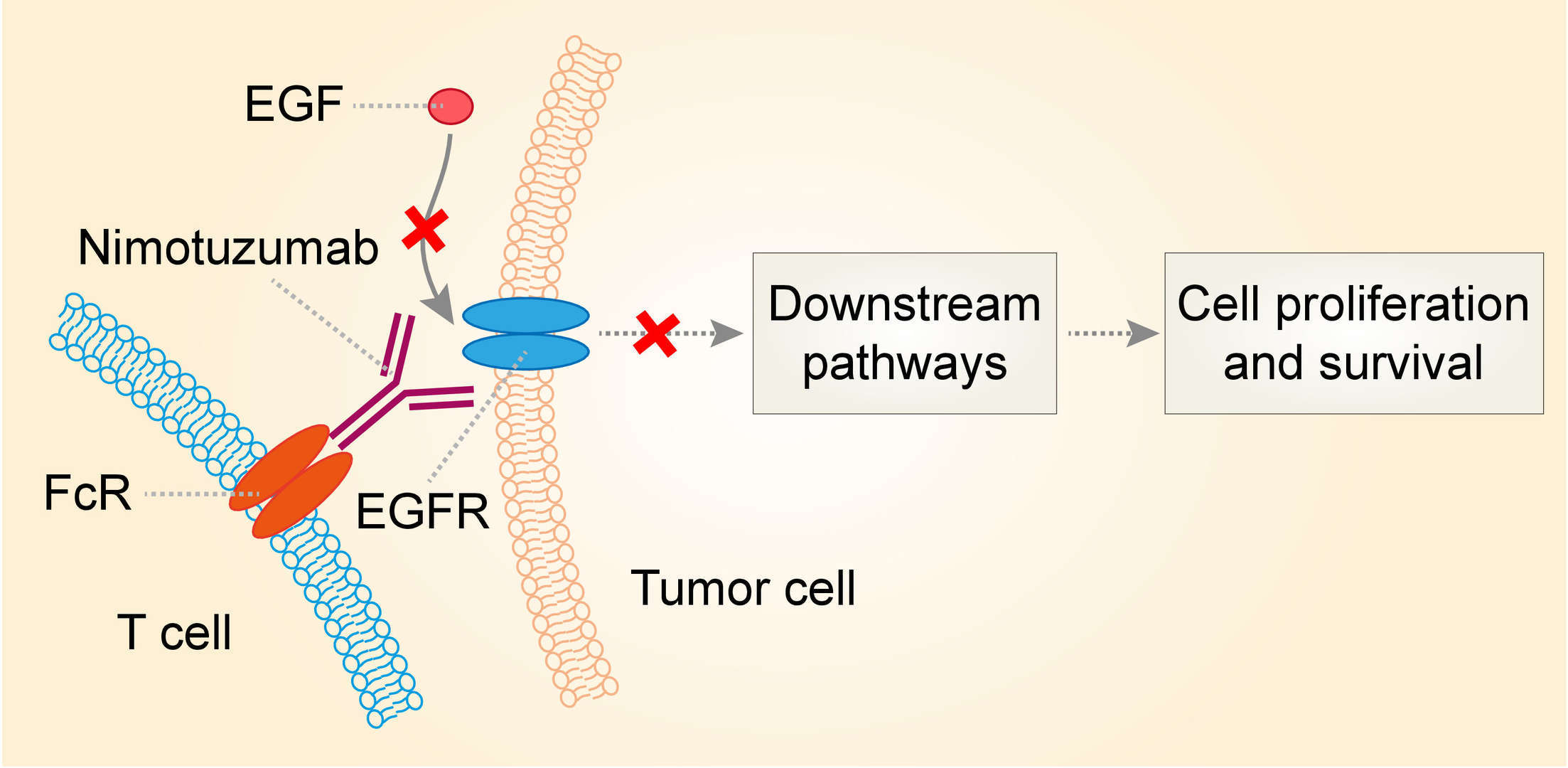 Fig.1 Mechanism of action of nimotuzumab
Fig.1 Mechanism of action of nimotuzumab
Clinical Projects of Nimotuzumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03554889 | Not yet recruiting | Advanced Cancer, ADCC, NK Cell Mediated Immunity, Nimotuzumab, Adaptive Transfer | Hangzhou Cancer Hospital | June 13, 2018 |
| NCT02577341 | Recruiting | Non-small-cell Lung Cancer | Sun Yat-sen University | October 16, 2015 |
| NCT00957086 | Recruiting | Carcinoma, Squamous Cell of Head and Neck | National Cancer Centre, Singapore | August 12, 2009 |
| NCT02705612 | Recruiting | Cervical Cancer | Fourth Military Medical University | March 10, 2016 |
| NCT02858206 | Recruiting | Esophageal Neoplasms | Chinese Academy of Medical Sciences | August 8, 2016 |
| NCT03469531 | Not yet recruiting | Neoplasms, Cervical Adenosquamous Cell Carcinoma, Cervical Squamous Cell Carcinoma in Situ, Stage IB/IIA/IIB/III/IVA Cervical Cancer, | Zhujiang Hospital | March 19, 2018 |
| NCT03557112 | Recruiting | Nasopharyngeal Carcinoma | Guiyang Medical University | June 14, 2018 |
| NCT03400592 | Recruiting | Stomach Neoplasms | Peking University | January 17, 2018 |
| NCT02947386 | Recruiting | EGFR Gene Mutation, Recurrent Non-Small Cell Lung Carcinoma, Stage IIIA/IIIB/IV Non-Small Cell Lung Cancer | Roswell Park Cancer Institute | October 27, 2016 |
| NCT02672241 | Recruiting | Childhood Brain Stem Neoplasm | Xinhua Hospital, Shanghai Jiao Tong University School of Medicine | February 3, 2016 |
| NCT03413579 | Recruiting | Cervical Cancer | El Kendi Pharmaceuticals Manufacturing Company | January 29, 2018 |
| NCT02945267 | Not yet recruiting | Unresectable Pancreatic Cancer | October 26, 2016 | |
| NCT02611700 | Recruiting | Esophageal Squamous Cell Carcinomas | Biotech Pharmaceutical Co., Ltd. | November 23, 2015 |
| NCT02428764 | Recruiting | Lung Cancer | Sun Yat-sen University | April 29, 2015 |
| NCT02409186 | Recruiting | Prosthesis Survival | Shandong Cancer Hospital and Institute | April 6, 2015 |
| NCT00702481 | Active, not recruiting | Head and Neck Cancer | National Cancer Centre, Singapore | June 20, 2008 |
| NCT03025958 | Recruiting | Nasopharyngeal Carcinoma | Zhejiang Cancer Hospital | January 20, 2017 |
What We Provide
Therapeutic Antibody
Nimotuzumab
We provide high-quality Nimotuzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Nimotuzumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

