

Obinutuzumab Overview
Introduction of Obinutuzumab
Obinutuzumab, previous known as afutuzumab, is a humanized IgG1κ type monoclonal antibody that targets CD20 antigen. CD20 has been found to play an important role in B cell-related diseases. This drug was originated by GlycArt Biotechnology AG and developed by Roche as a cancer treatment. Obinutuzumab has been used for the treatment of patients with previously untreated chronic lymphocytic leukemia (CLL) in combination with chlorambucil, and for the treatment of patients with patients with follicular lymphoma (FL) who have had prior therapy with the rituximab-containing regimen in combination with bendamustine. Obinutuzumab was approved by the U.S. Food and Drug Administration (FDA) under the trade name Gazyva in 2013, and as Gazyvaro by the European Medicines Agency in Europe in 2014. In addition, it also received the Therapeutic Goods Administration (TGA) approval and Canada health approval in 2014.
Mechanism of Action of Obinutuzumab
Obinutuzumab is the active substance in Gazyvaro and has been designed to recognize and attach to the protein CD20, which was expressed in 90% of B lymphoma cells and normal B lymphoid cells, but not in hematopoietic stem cells, primary B lymphoid cells, normal blood cells, and other normal tissues. Cancerous B-lymphocytes multiply too quickly and replace the normal cells in the bone marrow (where blood cells are made) and in lymph nodes in patients with chronic lymphocytic leukemia and follicular lymphoma. Thus, by binding to CD 20 molecular on the B-lymphocytes, obinutuzumab induces the human immune system to attack B-lymphocytes and clears it in the body. However, stem cells can re-differentiate into B lymphocytes after the body clears B lymphocytes after the treatment because there is no CD20 in stem cells. This allows the body to regain immunity. In contrast to the classic type I CD20 antibody, rituximab, obinutuzumab binds to type II CD20 antibodies. This binding allows obinutuzumab to have a much higher induction of antibody-dependent cytotoxicity and a higher direct cytotoxic effect compared with the classic CD20 antibodies.
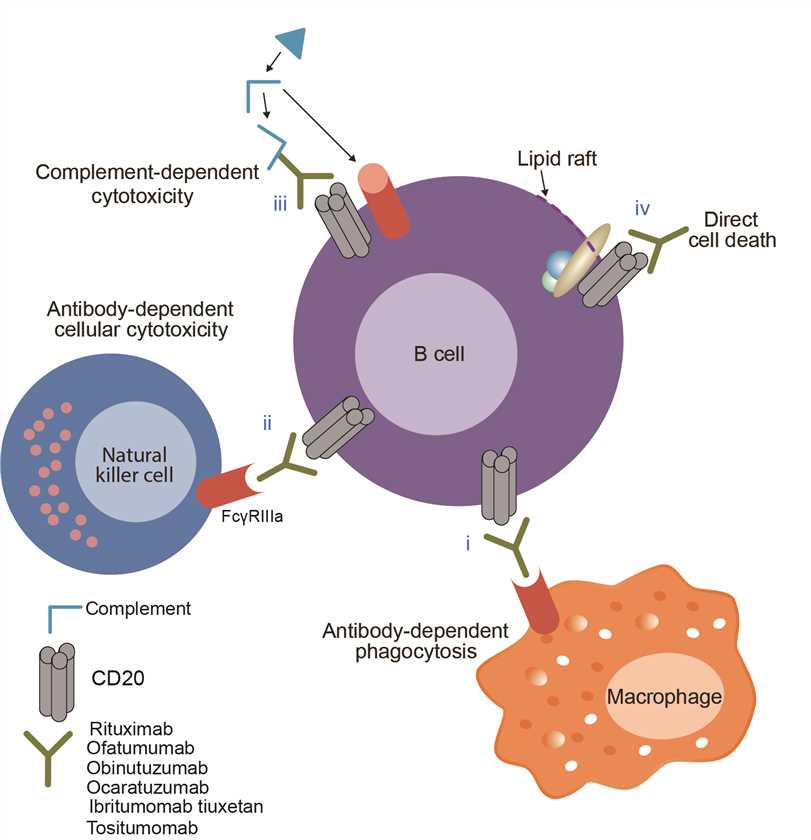
Fig.1 Mechanism of action of obinutuzumab
Table 1. Clinical Projects of Obinutuzumab *
| NCT ID | Status | Condition | Lead Sponsor | Update Time |
| NCT03059251 | Enrolling by invitation | Chronic Lymphocytic Leukemia; | Hoffmann-La Roche | October 30, 2018 |
| NCT03153514 | Active, not recruiting | Chronic Lymphocytic Leukemia; Richter`s Transformation; | German CLL Study Group | June 25, 2018 |
| NCT03311126 | Recruiting | Mantle Cell Lymphoma; Non-hodgkin Lymphoma; Non Hodgkin Lymphoma; | University of Wisconsin, Madison | April 25, 2018 |
| NCT03229382 | Recruiting | Mantle Cell Lymphoma; | Polish Lymphoma Research Group | October 17, 2018 |
| NCT02100852 | Active, not recruiting | Chronic Lymphocytic Leukemia; | TG Therapeutics, Inc. | March 20, 2018 |
| NCT02586051 | Active, not recruiting | Kidney Failure, Chronic; | Hoffmann-La Roche | October 31, 2018 |
| NCT03374137 | Recruiting | Chronic Lymphocytic Leukemia; Follicular Lymphoma; | Hoffmann-La Roche | April 25, 2018 |
| NCT02225275 | Recruiting | Leukemia; | M.D. Anderson Cancer Center | March 21, 2018 |
| NCT03322865 | Not yet recruiting | Marginal Zone Lymphoma; | Christian Buske | October 26, 2017 |
| NCT03332017 | Recruiting | Relapsed/Refractory Follicular Non-Hodgkin Lymphoma; | BeiGene | November 1, 2018 |
| NCT02315768 | Recruiting | Chronic Lymphocytic Leukemia; | University of California, San Diego | April 11, 2018 |
| NCT02611908 | Recruiting | Chronic Lymphocytic Leukemia; | Michael Choi | February 22, 2018 |
| NCT02867384 | Recruiting | Graft vs. Host Disease; | Dana-Farber Cancer Institute | July 2, 2018 |
| NCT03759184 | Not yet recruiting | Leukemia; Lymphocytic; Chronic;B-Cell; | National Cancer Institute (NCI) | November 30, 2018 |
| NCT02537613 | Recruiting | Chronic Lymphocytic Leukemia; | Dana-Farber Cancer Institute | October 18, 2018 |
| NCT03755947 | Not yet recruiting | B-Cell Chronic Lymphocytic Leukemia; B-Cell Chronic Lymphocytic Leukemia in Relapse (Diagnosis); | Grupo Cooperativo de Hemopatías Malignas | November 29, 2018 |
| NCT03580928 | Recruiting | Chronic Lymphocytic Leukemia (CLL); | Dana-Farber Cancer Institute | October 31, 2018 |
| NCT02877550 | Recruiting | Follicular Lymphoma; | Swiss Group for Clinical Cancer Research | July 11, 2018 |
| NCT02915224 | Recruiting | Chronic Lymphocytic Leukemia; | Hoffmann-La Roche | November 29, 2018 |
| NCT03198026 | Recruiting | Non-Hodgkin's Lymphoma; | Sidney Kimmel Cancer Center at Thomas Jefferson University | March 6, 2018 |
| NCT03679455 | Recruiting | Waldenstrom Macroglobulinemia; | Polish Myeloma Consortium | November 12, 2018 |
| NCT03410875 | Recruiting | Hairy Cell Leukemia; Leukemia; Leukemia, Hairy Cell; | Memorial Sloan Kettering Cancer Center | March 15, 2018 |
| NCT03113695 | Recruiting | Richter's Syndrome; CLL; | University of California, San Diego | March 27, 2018 |
| NCT01685892 | Active, not recruiting | Lymphocytic Leukemia, Chronic; | Genentech, Inc. | November 27, 2018 |
| NCT02498951 | Recruiting | CD20 Positive; Central Nervous System B-Cell Non-Hodgkin Lymphoma; | OHSU Knight Cancer Institute | September 20, 2018 |
| NCT02242942 | Active, not recruiting | Lymphocytic Leukemia, Chronic; | Hoffmann-La Roche | November 5, 2018 |
| NCT03145480 | Recruiting | Richter Syndrome; | Northwell Health | August 29, 2017 |
| NCT02320487 | Active, not recruiting | Chronic Lymphocytic Leukemia; | Genentech, Inc. | October 17, 2018 |
| NCT02992522 | Recruiting | B-Cell Lymphoma, Unclassifiable, With Features Intermediate Between Diffuse Large B-Cell Lymphoma and Burkitt Lymphoma; Grade 1 Follicular Lymphoma; Grade 2 Follicular Lymphoma; Grade 3a Follicular Lymphoma; Recurrent Burkitt Lymphoma; Recurrent Diffuse Large B-Cell Lymphoma; Recurrent Follicular Lymphoma; Recurrent Marginal Zone Lymphoma; Refractory Burkitt Lymphoma; Refractory Diffuse Large B-Cell Lymphoma; Refractory Follicular Lymphoma; Transformed Recurrent Non-Hodgkin Lymphoma; | Beth Christian | November 21, 2018 |
| NCT02736617 | Recruiting | Recurrent Mantle Cell Lymphoma; Refractory Mantle Cell Lymphoma; | OHSU Knight Cancer Institute | September 25, 2017 |
| NCT02417285 | Recruiting | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Non-Hodgkin; | Celgene | August 8, 2018 |
| NCT02871219 | Recruiting | Ann Arbor Stage II Grade 1 Follicular Lymphoma; Ann Arbor Stage II Grade 2 Follicular Lymphoma; Ann Arbor Stage III Grade 1 Follicular Lymphoma; Ann Arbor Stage III Grade 2 Follicular Lymphoma; Ann Arbor Stage IV Grade 1 Follicular Lymphoma; Ann Arbor Stage IV Grade 2 Follicular Lymphoma; Bulky Disease; Fatigue; Fever; Grade 3a Follicular Lymphoma; Night Sweats; Splenomegaly; Weight Loss; | M.D. Anderson Cancer Center | November 16, 2018 |
| NCT02612311 | Active, not recruiting | Chronic Lymphocytic Leukemia; | TG Therapeutics, Inc. | October 19, 2017 |
| NCT02529852 | Active, not recruiting | Lymphoma; | M.D. Anderson Cancer Center | April 19, 2018 |
| NCT02550652 | Active, not recruiting | Lupus Nephritis; | Hoffmann-La Roche | September 24, 2018 |
| NCT02569476 | Active, not recruiting | B-cell Lymphoid Malignancies; | BeiGene | July 4, 2018 |
| NCT02758665 | Active, not recruiting | Leukemia, Lymphocytic, Chronic; | University of Ulm | November 14, 2018 |
| NCT02962401 | Active, not recruiting | Waldenstrom Macroglobulinemia; | French Innovative Leukemia Organisation | September 3, 2018 |
| NCT02624986 | Active, not recruiting | Non-Hodgkin's Lymphoma; | Hoffmann-La Roche | October 23, 2018 |
| NCT02846623 | Recruiting | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma; | M.D. Anderson Cancer Center | November 14, 2018 |
| NCT02427451 | Active, not recruiting | Chronic Lymphocytic Leukemia; Refractory Chronic Lymphocytic Leukemia; | Kerry Rogers | November 21, 2017 |
| NCT03276468 | Recruiting | Follicular Lymphoma; Diffuse Large B Cell Lymphoma; Marginal Zone Lymphoma; Mucosa Associated Lymphoid Tissue; | The Lymphoma Academic Research Organisation | October 10, 2018 |
| NCT03010358 | Recruiting | Anemia; B-Cell Prolymphocytic Leukemia; Fatigue; Fever; Grade 1 Follicular Lymphoma; Grade 2 Follicular Lymphoma; Grade 3a Follicular Lymphoma; Hairy Cell Leukemia; Lymphadenopathy; Lymphocytosis; Lymphoplasmacytic Lymphoma; Mantle Cell Lymphoma; Marginal Zone Lymphoma; Night Sweats; Recurrent Chronic Lymphocytic Leukemia; Recurrent Small Lymphocytic Lymphoma; Refractory Chronic Lymphocytic Leukemia; Refractory Small Lymphocytic Lymphoma; Richter Syndrome; Splenomegaly; Thrombocytopenia; Weight Loss; | OHSU Knight Cancer Institute | October 2, 2018 |
| NCT03075696 | Recruiting | Non-Hodgkin's Lymphoma; | Hoffmann-La Roche | November 21, 2018 |
| NCT03039114 | Recruiting | Lymphoma; | Incyte Corporation | March 27, 2018 |
| NCT03701282 | Not yet recruiting | CCND1 Negative; Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma;t(11;14) Negative; | National Cancer Institute (NCI) | October 10, 2018 |
| NCT01059630 | Active, not recruiting | Non-Hodgkin's Lymphoma; | Genentech, Inc. | September 24, 2018 |
| NCT02983617 | Active, not recruiting | Chronic Lymphocytic Leukemia; | Gilead Sciences | September 11, 2018 |
| NCT03737981 | Not yet recruiting | Chronic Lymphocytic Leukemia; | National Cancer Institute (NCI) | November 12, 2018 |
| NCT02264574 | Active, not recruiting | Chronic Lymphocytic Leukemia; Small-Cell Lymphoma; | Pharmacyclics LLC. | January 6, 2017 |
| NCT02750670 | Active, not recruiting | Lymphoma, Non-Hodgkin's; | University Health Network, Toronto | September 26, 2018 |
| NCT02968563 | Recruiting | Chronic Lymphocytic Leukemia; | Gilead Sciences | November 7, 2018 |
| NCT02499003 | Active, not recruiting | Lymphoma, Non-Hodgkin; | Johannes Gutenberg University Mainz | November 29, 2018 |
| NCT02987400 | Recruiting | Lymphoma, Large B-Cell, Diffuse; | Arbeitsgemeinschaft medikamentoese Tumortherapie | May 17, 2017 |
| NCT01995669 | Recruiting | Lymphoma; | M.D. Anderson Cancer Center | August 6, 2018 |
| NCT03462719 | Recruiting | Leukemia, Lymphocytic, Chronic, B-Cell; | Janssen Research & Development, LLC | November 15, 2018 |
| NCT02296918 | Recruiting | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma; Prolymphocytic Leukemia; | Acerta Pharma BV | February 7, 2018 |
| NCT03135262 | Recruiting | Follicular Lymphoma; Lymphoma, Large B-Cell, Diffuse; | Hoffmann-La Roche | November 12, 2018 |
| NCT02475681 | Active, not recruiting | Chronic Lymphocytic Leukemia; | Acerta Pharma BV | May 31, 2017 |
| NCT02220842 | Active, not recruiting | Lymphoma; | Hoffmann-La Roche | October 23, 2018 |
| NCT01691898 | Active, not recruiting | Follicular Lymphoma; Diffuse Large B-Cell Lymphoma; | Genentech, Inc. | October 29, 2018 |
| NCT03492775 | Recruiting | Indolent Non-hodgkin Lymphoma; | Prof. Dr. Wolfgang Hiddemann | September 19, 2018 |
| NCT02631577 | Active, not recruiting | Lymphoma, Follicular; | Hoffmann-La Roche | September 24, 2018 |
| NCT03516617 | Recruiting | CCND1 Negative; Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma;t(11;14) Negative; | Mayo Clinic | September 27, 2018 |
| NCT02596971 | Active, not recruiting | Diffuse Large B-Cell Lymphoma, Lymphoma Follicular; | Hoffmann-La Roche | October 30, 2018 |
| NCT02729896 | Active, not recruiting | Lymphoma; | Hoffmann-La Roche | November 6, 2018 |
| NCT02629809 | Recruiting | Chronic Lymphocytic Leukemia; Immunoglobulin Heavy Chain Locus Variable Region Mutation; Small Lymphocytic Lymphoma; | M.D. Anderson Cancer Center | October 31, 2018 |
| NCT02689869 | Active, not recruiting | Indolent Non-Hodgkin Lymphoma; | Ludwig-Maximilians - University of Munich | September 19, 2018 |
| NCT02071225 | Active, not recruiting | Chronic Lymphocytic Leukemia; | Hoffmann-La Roche | October 30, 2018 |
| NCT02393157 | Recruiting | Non-Hodgkin Lymphoma; Burkitt Lymphoma; Diffuse Large B-Cell Lymphoma; Primary Mediastinal B-cell Lymphoma;CD20+ Lymphoblastic Lymphoma; Follicular Lymphoma, Grade III; | New York Medical College | December 22, 2017 |
| NCT02611323 | Recruiting | Non-Hodgkin's Lymphoma; | Hoffmann-La Roche | November 30, 2018 |
| NCT02896582 | Recruiting | Mantle Cell Lymphoma; | The Lymphoma Academic Research Organisation | March 7, 2018 |
| NCT01671904 | Active, not recruiting | Chronic Lymphocytic Leukemia; | Genentech, Inc. | August 10, 2018 |
| NCT03406156 | Recruiting | Chronic Lymphocytic Leukemia (CLL);Small Lymphocytic Lymphoma (SLL); | AbbVie | November 14, 2018 |
| NCT02229422 | Active, not recruiting | CLL; | University of California, San Diego | March 27, 2018 |
| NCT03529227 | Recruiting | Chronic Lymphocytic Leukemia; | Healthy Future | May 22, 2018 |
| NCT01332968 | Active, not recruiting | Non-Hodgkin's Lymphoma; | Hoffmann-La Roche | September 24, 2018 |
| NCT02600897 | Recruiting | Relapsed or Refractory Follicular Lymphoma, Relapsed or Refractory Diffuse Large B-Cell Lymphoma; | Hoffmann-La Roche | November 30, 2018 |
| NCT02453087 | Active, not recruiting | Non-Hodgkin's Lymphoma; | Hoffmann-La Roche | September 14, 2018 |
| NCT02406742 | Active, not recruiting | Leukemia, Lymphocytic, Chronic, B-Cell; | Celgene | October 2, 2018 |
| NCT01582776 | Active, not recruiting | Follicular Lymphoma Patients (Phase IB);Follicular and Agressive (DLBCL&MCL) B-cell Lymphoma Patients (Phase II); | The Lymphoma Academic Research Organisation | August 22, 2018 |
| NCT02257567 | Recruiting | Lymphoma; | Hoffmann-La Roche | November 16, 2018 |
| NCT03223610 | Recruiting | Lymphoma; Non-Hodgkin Lymphoma; Diffuse Large B-Cell Lymphoma; Burkitt Lymphoma; | National Cancer Institute (NCI) | September 7, 2018 |
| NCT01992653 | Active, not recruiting | Lymphoma, Non Hodgkin; | Genentech, Inc. | October 19, 2018 |
| NCT02558816 | Recruiting | Mantle Cell Lymphoma; | Nantes University Hospital | August 14, 2017 |
| NCT02950051 | Recruiting | Chronic Lymphocytic Leukemia; | German CLL Study Group | June 25, 2018 |
| NCT03467373 | Recruiting | B-Cell Lymphoma; Non-Hodgkin Lymphoma; | Hoffmann-La Roche | November 5, 2018 |
| NCT03269669 | Recruiting | Grade 1 Follicular Lymphoma; Grade 2 Follicular Lymphoma; Grade 3a Follicular Lymphoma; Recurrent Follicular Lymphoma; Refractory Follicular Lymphoma; | National Cancer Institute (NCI) | November 26, 2018 |
| NCT03341520 | Recruiting | Stage II Grade 1 Follicular Lymphoma; Stage II Grade 2 Follicular Lymphoma; Stage I Follicular Lymphoma Grade 1;Stage II Follicular Lymphoma Grade 2; | Heidelberg University | October 17, 2018 |
| NCT02445131 | Recruiting | Chronic Lymphocytic Leucemia; | German CLL Study Group | June 25, 2018 |
| NCT02324257 | Active, not recruiting | Solid Tumors; | Hoffmann-La Roche | November 20, 2018 |
| NCT02055820 | Active, not recruiting | Lymphoma, Non-Hodgkin; | Hoffmann-La Roche | March 27, 2018 |
| NCT01644253 | Active, not recruiting | Chronic Lymphocytic Leukemia; Peripheral T-cell Lymphoma; | Aptevo Therapeutics | November 28, 2018 |
| NCT03533283 | Recruiting | Non-Hodgkins Lymphoma; | Hoffmann-La Roche | November 26, 2018 |
| NCT02401503 | Active, not recruiting | Chronic Lymphocytic Leucemia; | German CLL Study Group | October 17, 2018 |
| NCT02457598 | Active, not recruiting | B-cell Malignancies; | Gilead Sciences | July 19, 2018 |
| NCT02345863 | Active, not recruiting | Chronic Lymphocytic Leucemia; | German CLL Study Group | June 25, 2018 |
| NCT02174172 | Active, not recruiting | Solid Cancers; | Hoffmann-La Roche | November 6, 2018 |
| NCT02320383 | Active, not recruiting | Chronic Lymphocytic Leucemia; | Munich Municipal Hospital | August 13, 2018 |
| NCT03432741 | Recruiting | Breast Adenocarcinoma; Recurrent Breast Carcinoma; Recurrent Hodgkin Lymphoma; Recurrent Mycosis Fungoides; Recurrent Non-Hodgkin Lymphoma; Recurrent Primary Cutaneous T-Cell Non-Hodgkin Lymphoma; Refractory Hodgkin Lymphoma; Refractory Mycosis Fungoides; Refractory Nodal Marginal Zone Lymphoma; Refractory Non-Hodgkin Lymphoma; Refractory Primary Cutaneous T-Cell Non-Hodgkin Lymphoma; Stage IV Breast Cancer AJCC v6 and v7; | Mayo Clinic | March 29, 2018 |
| NCT03671018 | Recruiting | B-cell Non-Hodgkin Lymphoma; | Hoffmann-La Roche | October 26, 2018 |
Table 2. Approved Drugs of obinutuzumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Gazyva | Chronic lymphocytic leukemia | Concentrate for solution | 25 mg/ml | Intravenous infusion | Genentech | November 1,2013 |

|
| Gazyvaro | Previously untreated chronic lymphocytic Leukaemia; Follicular lymphoma | Concentrate for solution | 25 mg/ml | Intravenous infusion | Roche Registration GmbH | July 22, 2014 |

|
| Gazyva | CD20 positive follicular lymphoma | Powder for injection | 25 mg/ml | Intravenous infusion | Chinese and Foreign Manufacturing Co., Ltd. | July 02, 2018 |

|
| Gazyva | Previously untreated chronic lymphocytic Leukaemia; Follicular lymphoma | Concentrate for solution | 25 mg/ml | Intravenous infusion | Hoffmann-La Roche Limited | November 28, 2014 |

|
| Gazyva | Chronic Lymphocytic Leukaemia | Concentrate for solution | 25 mg/ml | Intravenous infusion | Roche Products Pty Ltd | May 15, 2014 |

|
What We Provide
Therapeutic Antibody
Obinutuzumab
We provide high-quality Obinutuzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=obinutuzumab
https://www.ema.europa.eu/en/medicines/human/EPAR/gazyvaro
http://www.pmda.go.jp/PmdaSearch/iyakuSearch/
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

