

Ofatumumab Overview
Introduction of Ofatumumab
Ofatumumab, also known as HuMax-CD20, is an IgG1ĸ type fully human monoclonal antibody directed against CD20 antigen that expressed on normal B lymphocytes (pre-B- to mature B-lymphocyte) and on B-cell CLL. The molecular weight of ofatumumab is approximately 149 kDa. This drug was developed by Novartis Pharma AG and was designed for the treatment of chronic lymphocytic leukemia (CLL). In 2009, ofatumumab was approved by the U.S. Food and Drug Administration (FDA) for the treatment of chronic lymphocytic leukemia that is refractory to fludarabine and Arzerra. Ofatumumab was also conditional approved by Health Canada on August 2012, and by European Medicines Agency on April 19, 2010 for the treatment of chronic lymphocytic leukemia. In addition, this drug also received Therapeutic Goods Administration (TGA) approval and Japan Medical Devices Evaluation Center approval in 2013. In addition to CLL, ofatumumab also has also shown potential in treating follicular lymphoma, diffuse large B cell lymphoma, rheumatoid arthritis and relapsing remitting multiple sclerosis.
Mechanism of Action Ofatumumab
Ofatumumab is the active substance of Arzerra and has been designed to attach to a protein called CD20. Ofatumumab was found to inhibit the early-stage B lymphocyte activation. Thus, Ofatumumab became the first marketing application for an antibody produced by Genmab, as well as the first human monoclonal antibody which targets the CD20 molecule that will be available for patients with refractory CLL. Ofatumumab was designed to bind specifically to both the small and large extracellular loops of the CD20 molecule. Upon administration, the Fab domain of ofatumumab binds to the CD20 molecule and the Fc domain mediates immune effector functions. Binding of ofatumumab to CD20 stimulates the body’s immune system to attack the cancerous B-cells, helping to control the disease. In addition, by attaching to CD20, ofatumumab also could induce recruitment and activation of the complement pathway at the cell surface, leading to complement-dependent cytotoxicity and resultant lysis of tumour cells, and induce cell death through antibody-dependent cell-mediated cytotoxicity.
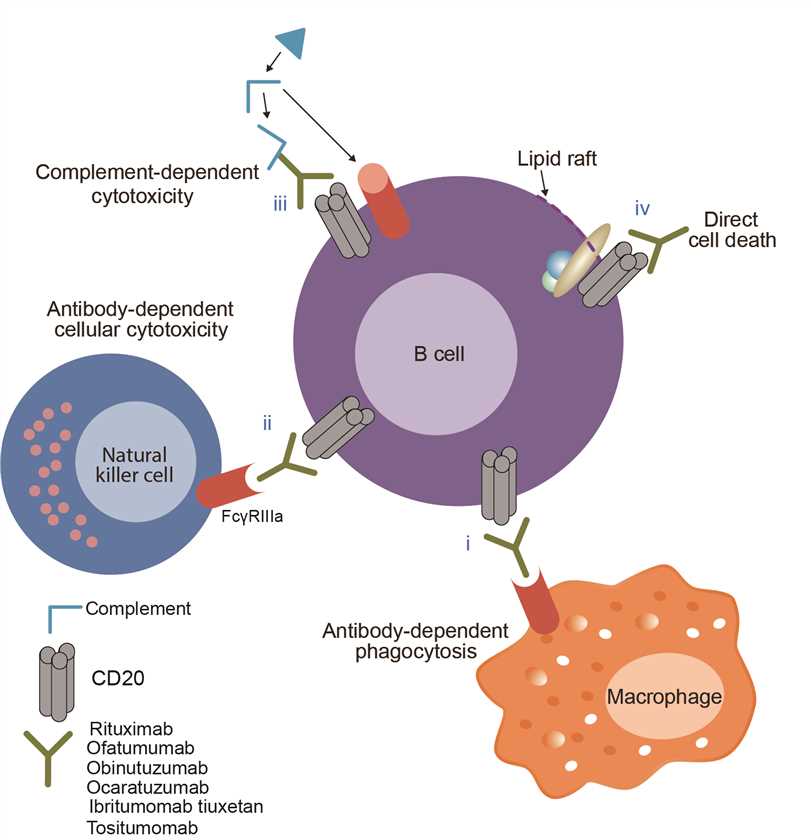
Fig.1 Mechanism of action of Ofatumumab
Table 1. Clinical Projects of Ofatumumab *
| NCT ID | Status | Condition | Lead Sponsor | Update Time |
| NCT03249714 | Recruiting | Relapsing Multiple Sclerosis | Novartis Pharmaceuticals | August 14, 2018 |
| NCT03650114 | Not yet recruiting | Relapsing Multiple Sclerosis | Novartis Pharmaceuticals | August 28, 2018 |
| NCT01680965 | Active, not recruiting | Chronic Graft Versus Host Disease | H. Lee Moffitt Cancer Center and Research Institute | November 6, 2018 |
| NCT01613300 | Active, not recruiting | B-Cell Lymphomas | Grupo Español de Linfomas y Transplante Autólogo de Médula Ósea | November 19, 2018 |
| NCT02388048 | Recruiting | Leukemia, Lymphoblastic, Chronic | Gruppo Italiano Malattie EMatologiche dell'Adulto | August 1, 2018 |
| NCT02394106 | Recruiting | Nephrotic Syndrome | Istituto Giannina Gaslini | May 4, 2017 |
| NCT01258933 | Active, not recruiting | Leukemia;Lymphoma | M.D. Anderson Cancer Center | September 14, 2018 |
| NCT01243190 | Active, not recruiting | Chronic Lymphocytic Leukemia | M.D. Anderson Cancer Center | September 25, 2018 |
| NCT01437709 | Active, not recruiting | Mantle Cell Lymphoma | Memorial Sloan Kettering Cancer Center | October 2, 2018 |
| NCT01444716 | Active, not recruiting | Chronic Lymphocytic Leukemia | M.D. Anderson Cancer Center | September 17, 2018 |
| NCT02710643 | Active, not recruiting | Follicular Lymphoma, Grade 1;Follicular Lymphoma, Grade 2;Follicular Lymphoma Grade 3A; | Fondazione Italiana Linfomi ONLUS | November 21, 2018 |
| NCT02394119 | Recruiting | Nephrotic Syndrome | Istituto Giannina Gaslini | May 4, 2017 |
| NCT02049515 | Enrolling by invitation | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma | Verastem, Inc. | October 16, 2018 |
| NCT02792218 | Active, not recruiting | Relapsing Multiple Sclerosis | Novartis Pharmaceuticals | October 4, 2018 |
| NCT02792231 | Recruiting | Relapsing Multiple Scelrosis | Novartis Pharmaceuticals | September 11, 2018 |
| NCT01077518 | Active, not recruiting | Lymphoma, Follicular | Novartis Pharmaceuticals | October 4, 2018 |
| NCT02614508 | Active, not recruiting | Recurrent Chronic Lymphocytic Leukemia; Recurrent Small Lymphocytic Lymphoma; Refractory Chronic Lymphocytic Leukemia; Refractory Small Lymphocytic Lymphoma; | Emory University | October 15, 2018 |
| NCT03280160 | Recruiting | Chronic Lymphocytic Leukemia | PETHEMA Foundation | September 13, 2017 |
| NCT02004522 | Active, not recruiting | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma | Verastem, Inc. | August 1, 2018 |
| NCT01458366 | Active, not recruiting | Non-Hodgkin's Lymphoma | Sidney Kimmel Cancer Center at Thomas Jefferson University | June 6, 2018 |
| NCT01578707 | Active, not recruiting | Relapsed or Refractory Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma | Pharmacyclics LLC. | May 25, 2018 |
| NCT01716208 | Active, not recruiting | Chronic Lymphocytic Leukemia | University of California, Davis | June 27, 2018 |
| NCT02689141 | Active, not recruiting | Chronic Lymphocytic Leukemia | German CLL Study Group | June 25, 2018 |
| NCT01190449 | Active, not recruiting | Lymphoma | Alliance for Clinical Trials in Oncology | July 25, 2018 |
| NCT01496976 | Active, not recruiting | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma | H. Lee Moffitt Cancer Center and Research Institute | November 6, 2018 |
| NCT01060384 | Active, not recruiting | Non-Hodgkin's Lymphoma | University of Nebraska | August 8, 2018 |
| NCT01497496 | Active, not recruiting | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma | H. Lee Moffitt Cancer Center and Research Institute | October 3, 2018 |
| NCT01329900 | Active, not recruiting | Lymphoma | M.D. Anderson Cancer Center | November 6, 2018 |
| NCT01363128 | Active, not recruiting | Leukemia | M.D. Anderson Cancer Center | November 26, 2018 |
| NCT01361711 | Active, not recruiting | Stage I Chronic Lymphocytic Leukemia; Stage II Chronic Lymphocytic Leukemia; Stage III Chronic Lymphocytic Leukemia; Stage IV Chronic Lymphocytic Leukemia; | Northwestern University | February 16, 2018 |
| NCT02199184 | Recruiting | Leukemia | M.D. Anderson Cancer Center | December 2, 2017 |
| NCT03560739 | Recruiting | Multiple Sclerosis | Novartis Pharmaceuticals | November 29, 2018 |
| NCT01465334 | Active, not recruiting | CLL;SLL | Dana-Farber Cancer Institute | June 29, 2018 |
| NCT01762202 | Active, not recruiting | B-cell Lymphoid Leukemia; Young Patients | Gruppo Italiano Malattie EMatologiche dell'Adulto | October 15, 2018 |
| NCT01527149 | Active, not recruiting | Stage I Mantle Cell Lymphoma; Stage II Contiguous Mantle Cell Lymphoma Stage II Non-Contiguous Mantle Cell Lymphoma Stage III Mantle Cell Lymphoma; Stage IV Mantle Cell Lymphoma; | Roswell Park Cancer Institute | January 29, 2018 |
| NCT01145209 | Active, not recruiting | Small Lymphocytic Lymphoma; CLL (Chronic Lymphocytic Leukemia) | National Heart, Lung, and Blood Institute (NHLBI) | August 17, 2018 |
| NCT01555541 | Recruiting | Diffuse Large Cell Lymphoma Relapsed/Refractory | C. Babis Andreadis | June 9, 2017 |
| NCT02877303 | Recruiting | Leukemia; Acute Lymphoblastic Leukemia | M.D. Anderson Cancer Center | November 6, 2018 |
| NCT03488225 | Recruiting | Acute Lymphocytic Leukemia | M.D. Anderson Cancer Center | November 22, 2018 |
| NCT03136146 | Recruiting | Hematopoietic/Lymphoid Cancer; Acute Lymphoblastic Leukemia; Lymphoblastic Lymphoma; Burkitt Leukemia/Lymphoma; | M.D. Anderson Cancer Center | September 5, 2018 |
| NCT02361346 | Recruiting | Non-Hodgkin's B-cell Lymphoma; Leukemia, Lymphocytic, Chronic, B-CellSmall Lymphocytic Leukemia; | Molecular Templates, Inc. | November 5, 2018 |
Table 2. Approved Drugs of Ofatumumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Arzerra | Chronic lymphocytic leukemia | Injection | 100mg /5ml | Intravenous Infusion | Glaxo Grp Ltd | October 26, 2009 |

|
| Arzerra | Chronic lymphocytic leukaemia | Concentrate for solution | 20 mg / ml | Intravenous infusion | Novartis Europharm Ltd | April 19, 2010 |

|
| Arzerra | Recurrent or refractory CD20 positive chronic lymphocytic leukemia | Concentrate for solution | 50ml/vial | Intravenous infusion | Novartis Pharma Co., Ltd. | 25th March, 2013 |

|
| Arzerra | Chronic lymphocytic leukaemia | Solution | 100 mg / 5 ml | Intravenous infusion | Novartis Pharmaceuticals Canada Inc | August 13, 2012 |

|
| Arzerra | Chronic lymphocytic leukaemia | Solution | 1000 mg / 50 ml | Intravenous infusion | Novartis Pharmaceuticals Canada Inc | August 13, 2012 |

|
| Arzerra | Chronic lymphocytic leukaemia | Injection | 20 mg/mL | Intravenous infusion | Novartis Pharmaceuticals Australia Pty Ltd | February 11, 2013 |

|
What We Provide
Therapeutic Antibody
Ofatumumab
We provide high-quality Ofatumumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=Ofatumumab
** Information presented in the table were collected from the following website:
https://search.tga.gov.au/s/search.html?collection=tga-websites-web&query=Ofatumumab
https://www.ema.europa.eu/en/medicines/human/EPAR/arzerra
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

