

Omalizumab Overview
Introduction of Omalizumab
Omalizumab is a recombinant DNA-derived humanized IgG1κ monoclonal antibody (mAb) designed for the treatment of asthma and urticaria. It specifically binds to free human immunoglobulin E (IgE) in the blood and interstitial fluid and to membrane-bound form of IgE on the surface of mIgE-expressing B lymphocytes. Unlike an ordinary anti-IgE antibody, it does not bind to IgE that is already bound by the high affinity IgE receptor (FcεRI) on the surface of mast cells, basophils, and antigen-presenting dendritic cells. Omalizumab is a glycosylated IgG1 monoclonal antibody produced by cells of an adapted Chinese hamster ovary (CHO) cell line. The antibody molecules are secreted by the host cells in a cell culture process employing large-scale bioreactors. At the end of culturing, the IgG contained in the medium is purified by an affinity-column using Protein A as the adsorbent, followed by chromatography steps, and finally concentrated by UF/DF (paired ultra-filtration/depth filtration). Omalizumab is manufactured at the Novartis' Huningue manufacturing site (France) through a partnership agreement with Genentech. Omalizumab was for several years provided only in a dry powder formulation, which requires the reconstitution with a prepacked solvent with the help of a shaker at the treating clinician’s office before injection. A prefilled syringe liquid formulation has become available in many countries.
Mechanism of Action of Omalizumab
Omalizumab is a recombinant humanized monoclonal antibody that was designed to bind to IgE on the Fc (constant fragment) portion, C epsilon 3 locus, in the same domain where IgE is bound to FcεRI. This drug was synthetized with the aim of sequestering free IgE and reducing allergic inflammation. The drug is administered subcutaneously and is absorbed slowly. The peak of serum concentration is reached after 7-8 days and it is eliminated via the reticuloendothelial system, having a half-life of around 26 days. It has been accepted for a long time that omalizumab acts on the free IgE and may abolish the binding of IgE to FcεRI+ or FcεRII+ (CD23) cells, B-cells, dendritic cells (DC), eosinophils (Eo), and monocytes. Interestingly, in recent years, the drug's action has been shown to go further, dissociating bound IgE from the IgE-FcεRI complex. Thus, at a physiological concentration range, omalizumab may accelerate the dissociation of the preformed IgE-FcεRI complex on the surfaces of mast cells and basophils in addition to its ability to neutralize the free IgE, leading to an impairment of the IgE-inflammatory signaling cascade. Moreover, the density of FcεRI expression on basophils, mast cells, and dendritic cells falls notably in patients receiving anti-IgE treatment within the first week of omalizumab application. This may be because the IgE stabilizes the receptor on the cell surface and prevents its internalization; thus, a reduction of the immunoglobulin leads to a decrease in receptor expression. All these events make these cells unresponsive to IgE triggering and reduce symptoms such as inflammation, edema, and pruritus. Finally, this leads to a reduction in MC/BS numbers. Interestingly, the reduction in FcεRI expression has also been demonstrated in dendritic cells. Likewise, as a complementary mechanism, it has been proposed that omalizumab -IgE complexes can bind to antigens and act as competitive inhibitors.
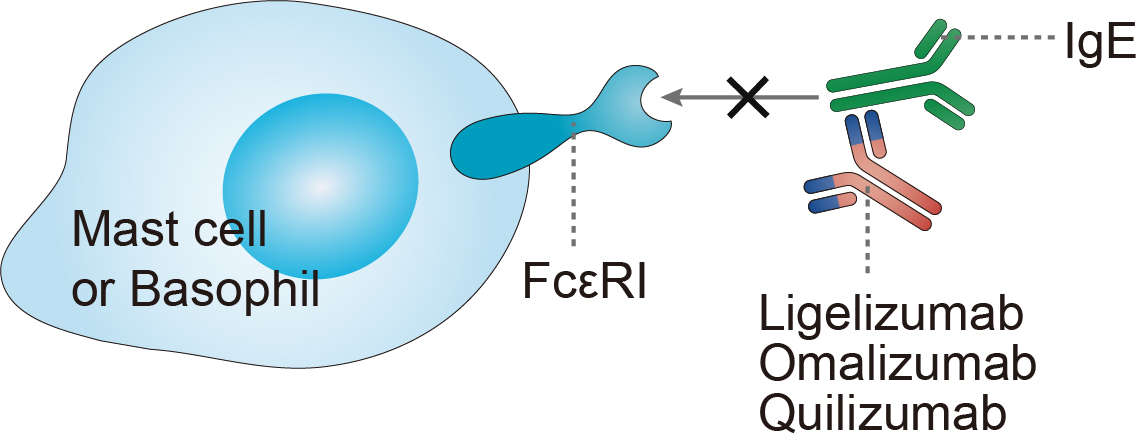
Fig 1. Mechanism of Action of Omalizumab
Table 1. Clinical Projects of Omalizumab *
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02266355 | Recruiting | Colon Cancer | Yale University | October 17, 2014 |
| NCT01460862 | Active, not recruiting | Asthma | Novartis Pharmaceuticals | October 27, 2011 |
| NCT03480815 | Enrolling by invitation | Severe Allergic Asthma | Chang Gung Memorial Hospital | March 29, 2018 |
| NCT02658877 | Recruiting | Asthma | New York University School of Medicine | January 20, 2016 |
| NCT03328897 | Recruiting | Chronic Spontaneous Urticaria | Novartis Pharmaceuticals | November 1, 2017 |
| NCT00890162 | Active, not recruiting | Anaphylaxis, Hypotension, Bronchospasm, Angioedema | National Institute of Allergy and Infectious Diseases (NIAID) | April 29, 2009 |
| NCT03727971 | Recruiting | Asthma; Infertility, Female | Bispebjerg Hospital | November 1, 2018 |
| NCT03361111 | Recruiting | Severe Asthma | Medical University of Warsaw | December 4, 2017 |
| NCT03478930 | Recruiting | Nasal Polyps, Chronic Rhinosinusitis | Hoffmann-La Roche | March 27, 2018 |
| NCT03280550 | Active, not recruiting | Nasal Polyps, Chronic Rhinosinusitis | Hoffmann-La Roche | September 12, 2017 |
| NCT03280537 | Active, not recruiting | Nasal Polyps, Chronic Rhinosinusitis | Hoffmann-La Roche | September 12, 2017 |
| NCT03476109 | Not yet recruiting | Severe Asthma | Cliniques universitaires Saint-Luc- Université Catholique de Louvain | March 23, 2018 |
| NCT02402231 | Active, not recruiting | Peanut Allergy | Caroline Nilsson | March 30, 2015 |
| NCT02966314 | Recruiting | Idiopathic Angioedema | University of Wisconsin, Madison | November 17, 2016 |
| NCT02879006 | Recruiting | Food Allergy | Xiu-Min Li | August 25, 2016 |
| NCT01781637 | Active, not recruiting | Peanut Allergy, Food Allergy | Boston Children’s Hospital | February 1, 2013 |
| NCT02300701 | Active, not recruiting | Atopic Eczema, Atopic Dermatitis, Child | Guy's and St Thomas' NHS Foundation Trust | November 25, 2014 |
| NCT02124226 | Not yet recruiting | Low Dose Methotrexate in Severe Chronic Asthma | Universita degli Studi di Catania | April 28, 2014 |
| NCT03181009 | Recruiting | Food Allergy | Kari Christine Nadeau | June 8, 2017 |
Table 2. Approved Drugs of Omalizumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Xolair | Asthma; Urticaria | Powder for injection | 150 mg | Subcutaneous injection | Genentech | April 7, 2006 |

|
| Xolair | Asthma; Urticaria | Solution for injection | 150 mg / mL | Subcutaneous injection | Genentech | April 7, 2006 |

|
| Xolair | Asthma; Urticaria | Powder for injection | 75 mg | Subcutaneous injection | Novartis Europharm Limited | October 20, 2005 |

|
| Xolair | Asthma; Urticaria | Powder for injection | 150 mg | Subcutaneous injection | Novartis Pharmaceuticals Canada Inc | February 23, 2005 |

|
| Xolair | Asthma; Urticaria | Solution for injection | 150 mg / mL | Subcutaneous injection | Novartis Pharmaceuticals Canada Inc | June 28, 2017 |

|
| Xolair | Asthma; Urticaria | Powder for injection | 150 mg | Subcutaneous injection | Novartis Pharmaceuticals Australia Pty Ltd | June 13, 2002 |

|
| Xolair | Asthma; Urticaria | Solution for injection | 150 mg / mL | Subcutaneous injection | Novartis Pharmaceuticals Australia Pty Ltd | August 16, 2013 |

|
| Xolair | Asthma; Urticaria | Solution for injection | 75 mg | Subcutaneous injection | Novartis Pharmaceuticals Australia Pty Ltd | August 19, 2013 |

|
| Xolair | Asthma; Urticaria | Powder for injection | 150 mg | Subcutaneous injection | Novartis Pharma K.K. | August 20, 2013 |

|
What We Provide
Therapeutic Antibody
Omalizumab
We provide high-quality Omalizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Omalizumab
** Information presented in the table were collected from the following website:
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=82744
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=201126
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=201124
https://www.ema.europa.eu/en/medicines/human/EPAR/xolair
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i= 103976
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=74602
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=94711
http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/2290400D1
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

