

Pamrevlumab Overview
Introduction of Pamrevlumab
Pamrevlumab is a fully human monoclonal antibody (mAb) developed by FibroGen. It inhibits the activity of connective tissue growth factor (CTGF), a critical mediator in the progression of fibrosis and related serious diseases. Many of these diseases, including idiopathic pulmonary fibrosis (IPF), pancreatic cancer, and Duchenne muscular dystrophy (DMD) have few, if any, effective therapeutic options. In Phase 1 and Phase 2 clinical studies involving more than 450 subjects, pamrevlumab was well tolerated across a wide range of doses with no dose-limiting toxicities observed to date. Early on, it was observed that pamrevlumab reversed fibrosis in a highly predictive animal model of lung fibrosis. In Phase 2 IPF clinical studies, pamrevlumab demonstrated the potential for stabilization of disease and, for the first time in human studies, reversal of lung fibrosis in some patients. Positive results were reported from the Phase 2 randomized, double-blind, placebo-controlled study and a double-blind, active-controlled combination sub-study for the treatment of IPF in the third quarter of 2017. In September 2018, pamrevlumab received fast track designation by the U.S. Food and Drug Administration (FDA) for the treatment of IPF. Besides IPF, the San Francisco-based company also will test pamrevlumab in Phase 3 stage in pancreatic cancer. The therapy is currently being tested in a Phase 2 study of Duchenne muscular dystrophy (NCT02606136). Pamrevlumab was granted orphan drug designation in both IPF and pancreatic cancer. In March 2018, the antibody therapy received the FDA’s fast track designation for the treatment of locally advanced unresectable pancreatic cancer.
Mechanism of Action of Pamrevlumab
CTGF, a 36-38kDa cysteine-rich peptide, is predominantly identified in endothelial cells, fibroblasts, cartilaginous cells, smooth muscle cells, and some cancer cell lines. CTGF is believed to be a multifunctional signaling modulator involved in a wide variety of biologic or pathologic processes, such as angiogenesis, osteogenesis, renal disease, skin disorders, and tumor development. There are at least 21 different human tumors or cancers that have been found to have CTGF expression, signifying its influence on the biology and progression of cancer. Of particular interest is the fact that CTGF is found to be expressed in human tumor cells or surrounding stromal cells, including acute lymphoblastic leukemia (ALL), breast cancer cells, cervical cancer, chondrosarcoma, cutaneous fibrohistiocytic and vascular tumors, esophageal cancer, gastric cancer, glioblastoma and gliomas, hepatocellular carcinomas, laryngeal squamous cell carcinoma (SCC), non-small cell lung cancer (NSCLC), melanoma, myofibroblastic tumors, oral SCC, ovarian cancer, pancreatic cancer, prostate cancer, rhabdomyosarcoma, and Wilms tumor. One of the main functions of CTGF is to promote cell adhesion through a unique integrin and heparin sulfate proteoglycan dependent mechanism. The carboxyl terminal 10 kDa of CTGF, which binds heparin, has been shown to be sufficient to promote cell adhesion. This fragment also promotes fibroblast proliferation. The integrins through which CTGF promotes adhesion vary depending on the cell type; for example, CTGF promotes adhesion to human foreskin fibroblasts through integrin a6b1, to human platelets through integrin aIIb3, to endothelial cells through integrin avb3, and to blood monocytes through integrin aMb2. High levels of CTGF expression has been detected in many fibrotic lesions, indicating its role in promoting fibrosis. CTGF exhibits mitogenic and chemotactic effects on fibroblasts and is also reported to enhance the mRNA expression of a1(I) collagen, fibronectin, and a5 integrin in fibroblasts. The finding that TGF-β increases CTGF synthesis and that TGF-β and CTGF share many functions in common, is consistent with the hypothesis that CTGF is a downstream mediator of TGF-β. In endothelial cells, CTGF mediates several functions such as proliferation, migration, differentiation, and survival, leading to enhanced angiogenesis. It also induces chondrocyte proliferation and differentiation.
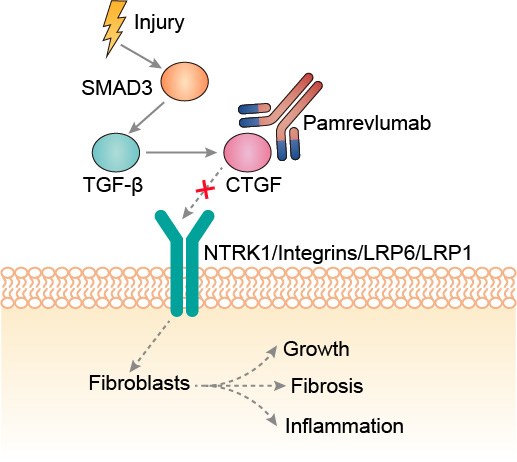 Fig.1 Mechanism of action of pamrevlumab
Fig.1 Mechanism of action of pamrevlumab
Table 1. Clinical Projects of Pamrevlumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02606136 | Active, not recruiting | Duchenne Muscular Dystrophy | FibroGen | November 17, 2015 |
What We Provide
Therapeutic Antibody
Pamrevlumab
We provide high-quality Pamrevlumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Pamrevlumab
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

