

Pertuzumab Overview
Introduction of Pertuzumab
Pertuzumab (also called 2C4, trade name Perjeta) is a humanized (from mouse) monoclonal antibody (mAb) used in combination with trastuzumab and docetaxel for the treatment of metastatic human epidermal growth factor receptor 2 (HER2)-positive breast cancer; it also used in the same combination as a neoadjuvant in early HER2-positive breast cancer. It was discovered and developed by Genentech, a subsidiary of Roche, and was first approved in 2012. It is manufactured recombinantly in Chinese hamster ovary (CHO) cells. The monoclonal antibody 2C4 appears to have first been published in 1990 by scientists from Genentech. By 2003 Genentech understood that 2C4 prevented HER2 dimerizing with other HER receptors and had begun Phase I trials, aiming for a broad range of cancers, not just one’s overexpressing HER2. It was the first known HER dimerization inhibitor. In 2005 Genentech presented poor results of Phase II trials of pertuzumab as a single agent in prostate, breast, and ovarian cancers, and said that it intended to continue developing it in combination with other drugs for ovarian cancer. In 2007 Genentech dropped the trade name Omnitarg. In 2012 the results were published of the CLEOPATRA trial, a randomized placebo-controlled Phase III trial of pertuzumab in combination with trastuzumab and docetaxel in HER2-positive metastatic breast cancer. Pertuzumab received US Food and Drug Administration (FDA) approval for the treatment of HER2-positive metastatic breast cancer later that year. The FDA approved the neoadjuvant indication in 2013. Pertuzumab was approved in Europe in 2013.
Mechanism of Action of Pertuzumab
The human epidermal growth factor receptor (HER) family comprises four type I transmembrane tyrosine kinase receptors (EGRFR or HER1, HER2, HER3 and HER4) with key roles in cell growth, proliferation, survival and oncogenesis. Each receptor consists of an extracellular segment with four domains involved in ligand binding and receptor dimerization, a transmembrane region, and an intracellular domain with kinase activity. Normally a bound ligand is necessary for receptor dimerization, except for the HER2 receptor that is constitutively available for dimerization and forms heterodimers with other ligand-activated HER receptors or homodimers in HER2- overexpressing cells. Pertuzumab is a humanized monoclonal antibody that binds the extracellular domain II of HER2. As a result, pertuzumab inhibits the ligand-mediated dimerization of HER2 by steric hindrance, inactivating multiple downstream signaling networks including the mitogen-activated protein kinase cascade (RAS/RAF/MEK/ERK) and the phosphoinositide 3-kinase (PI3K/AKT/mTOR) pathway. Complementary, trastuzumab acts mainly by inhibiting the ligand-independent HER2 signaling, preventing HER2 constitutive activation by extracellular domain cleavage. As it binds to the extracellular domain of HER2, pertuzumab can also induce an antibody-mediated immune effector function as seen with trastuzumab, but does not block HER2 shedding, furthermore, there is a growing evidence on the role of pertuzumab in increasing the trastuzumab-induced antibody-dependent cellular cytotoxicity (ADCC). The ADCC is a cell-mediated immune response triggered by antibodies that engage an immune system effector cell (typically natural killer cells) to lyse a target cell and releases cytokines such as interferon γ (IFNγ).
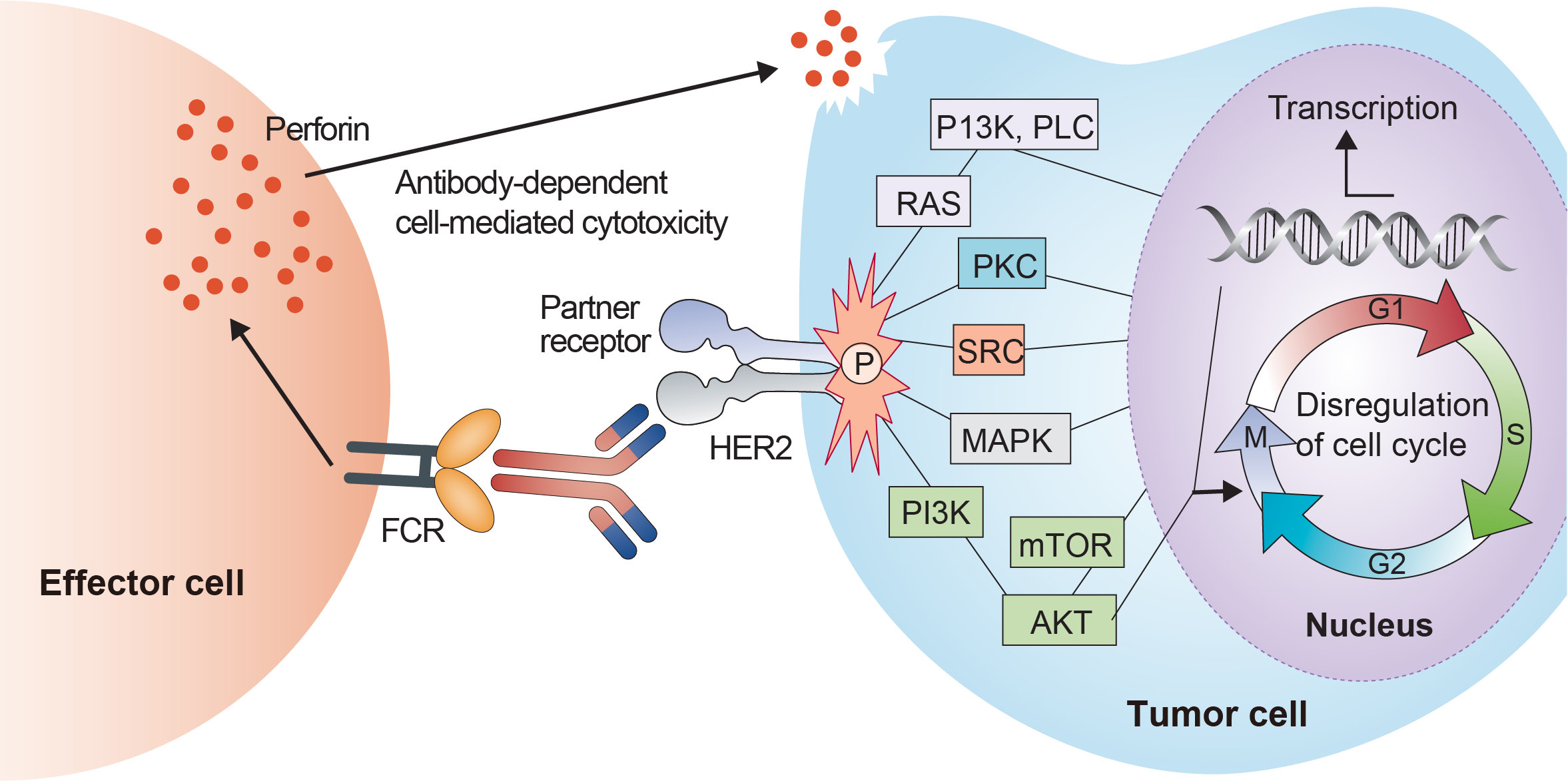 Fig.1 Mechanism of action of Pertuzumab
Fig.1 Mechanism of action of Pertuzumab
Table 1. Clinical Projects of Pertuzumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT02252887 | Active, not recruiting | Metastatic Her2-Positive Breast Cancer | Memorial Sloan Kettering Cancer Center | September 30, 2014 |
| NCT02625441 | Recruiting | Breast Cancer | Helsinki University Central Hospital | December 9, 2015 |
| NCT03025711 | Recruiting | Breast Neoplasms | BELEN RUIZ-ANTORAN | January 19, 2017 |
| NCT03304080 | Recruiting | Breast Neoplasms, Breast Diseases | Icahn School of Medicine at Mount Sinai | October 6, 2017 |
| NCT02436993 | Recruiting | Breast Cancer | University of California, Irvine | May 7, 2015 |
| NCT02642458 | Active, not recruiting | Breast Carcinoma | University Hospital Tuebingen | December 30, 2015 |
| NCT03101748 | Recruiting | Metastatic Breast Cancer | M.D. Anderson Cancer Center | April 5, 2017 |
| NCT03417544 | Recruiting | Malignant Neoplasm of Breast | Nancy Lin, MD | January 31, 2018 |
| NCT02689921 | Active, not recruiting | Her2-positive Metastatic Breast Cancer, Central Nervous System Metastases | Midwestern Regional Medical Center | February 24, 2016 |
| NCT02326974 | Recruiting | Breast Neoplasms | Dana-Farber Cancer Institute | December 30, 2014 |
| NCT01835236 | Active, not recruiting | HER-2 Positive Breast Cancer, Breast Cancer, Stage II Breast Cancer, Stage III Breast Cancer | Swiss Group for Clinical Cancer Research | April 18, 2013 |
| NCT03135171 | Recruiting | Metastatic Breast Cancer | University of Michigan Cancer Center | May 1, 2017 |
| NCT01730833 | Recruiting | Breast Cancer | City of Hope Medical Center | November 21, 2012 |
| NCT03199885 | Not yet recruiting | Breast Cancer | National Cancer Institute (NCI) | June 27, 2017 |
| NCT03264547 | Recruiting | HER2-positive Breast Cancer, Recurrent Breast Cancer, Stage IIA Breast Cancer, Stage IIB Breast Cancer, Stage IIIA Breast Cancer, Stage IIIB Breast Cancer, Stage IIIC Breast Cancer, Stage IV Breast Cancer, Breast Adenocarcinoma, Inflammatory Breast Carcinoma | Japan Breast Cancer Research Group | August 29, 2017 |
| NCT02536339 | Active, not recruiting | Breast Adenocarcinoma, HER2/Neu Positive, Recurrent Breast Carcinoma, Stage III Breast Cancer AJCC v7, Stage IIIA Breast Cancer AJCC v7, Stage IIIB Breast Cancer AJCC v7, Stage IIIC Breast Cancer AJCC v7, Stage IV Breast Cancer AJCC v6 and v7 | Genentech, Inc. | August 31, 2015 |
| NCT02139358 | Active, not recruiting | Breast Cancer | H. Lee Moffitt Cancer Center and Research Institute | May 15, 2014 |
| NCT03125928 | Recruiting | Metastatic Breast Cancer | Fox Chase Cancer Center | April 24, 2017 |
| NCT02205047 | Recruiting | Breast Cancer | European Organisation for Research and Treatment of Cancer - EORTC | July 31, 2014 |
| NCT03161353 | Recruiting | Her2-positive Breast Cancer | MedSIR | May 19, 2017 |
| NCT01855828 | Active, not recruiting | Malignant Neoplasm of Stomach, Malignant Neoplasm of Cardio-esophageal Junction of Stomach, Epidermal Growth Factor Receptor (EGFR) Protein Overexpression | Yale University | May 17, 2013 |
| NCT03365882 | Recruiting | HER2 Positive Breast Carcinoma, Stage IIIA Breast Cancer, Stage IIIB Breast Cancer, Stage IIIC Breast Cancer | Southwest Oncology Group | December 7, 2017 |
| NCT03619044 | Not yet recruiting | Her2-positive Locally Advanced or Metastatic Breast Cancer | Institut Claudius Regaud | August 7, 2018 |
| NCT02073487 | Active, not recruiting | Breast Cancer | Jenny C. Chang, MD | February 27, 2014 |
| NCT03674112 | Not yet recruiting | Her2-Positive Breast Cancer | Hoffmann-La Roche | September 17, 2018 |
| NCT02229149 | Active, not recruiting | Elderly Metastatic Breast Cancer Population | US Oncology Research | August 29, 2014 |
| NCT03225937 | Recruiting | Colon Adenocarcinoma, ERBB2 Gene Amplification, Rectal Adenocarcinoma, Recurrent Colon Carcinoma, Recurrent Rectal Carcinoma, Stage III Colon Cancer AJCC v7, Stage III Rectal Cancer AJCC v7, Stage IIIA Colon Cancer AJCC v7, Stage IIIA Rectal Cancer AJCC v7, Stage IIIB Colon Cancer AJCC v7, Stage IIIB Rectal Cancer AJCC v7, Stage IIIC Colon Cancer AJCC v7, Stage IIIC Rectal Cancer AJCC v7, Stage IV Colon Cancer AJCC v7, Stage IV Rectal Cancer AJCC v7, Stage IVA Colon Cancer AJCC v7, Stage IVA Rectal Cancer AJCC v7, Stage IVB Colon Cancer AJCC v7, Stage IVB Rectal Cancer AJCC v7 | Fondazione del Piemonte per l'Oncologia | July 21, 2017 |
| NCT01875666 | Active, not recruiting | Her2-positive Breast Cancer | UNC Lineberger Comprehensive Cancer Center | June 12, 2013 |
| NCT02581462 | Active, not recruiting | Breast Cancer | IKF Klinische Krebsforschung GmbH at Krankenhaus Nordwest | October 21, 2015 |
| NCT03272477 | Recruiting | Her2-Positive Early Breast Cancer | Palleos Healthcare GmbH | September 5, 2017 |
| NCT01817452 | Recruiting | Breast Neoplasms, Malignant Tumor of the Breast | West German Study Group | March 25, 2013 |
| NCT02910219 | Recruiting | Metastatic Colorectal Cancer | Georgetown University | September 21, 2016 |
| NCT02344472 | Recruiting | Breast Neoplasms | Prof. W. Janni | January 26, 2015 |
| NCT03595592 | Not yet recruiting | Stomach Cancer, Gastroesophageal Junction Cancer | Fondazione Michelangelo | July 23, 2018 |
| NCT02993198 | Recruiting | Solid Tumor | Northwestern University | December 15, 2016 |
| NCT03414658 | Recruiting | Early Breast Cancer | Ian E. Krop, MD, PhD | January 30, 2018 |
| NCT02339532 | Recruiting | Breast Cancer | UNICANCER | January 15, 2015 |
| NCT01376505 | Recruiting | Breast Neoplasms | Pravin Kaumaya | June 20, 2011 |
| NCT02789657 | Recruiting | HER-2 Positive Breast Cancer | William Sikov | June 3, 2016 |
| NCT03460067 | Recruiting | Gastric Cancer | Melissa Mitchell | March 9, 2018 |
| NCT01850628 | Recruiting | Breast Neoplasms | NSABP Foundation Inc | May 9, 2013 |
| NCT01276041 | Active, not recruiting | Breast Cancer | Memorial Sloan Kettering Cancer Center | January 13, 2011 |
| NCT01796197 | Active, not recruiting | Estrogen Receptor Positive, HER2/Neu Positive, Progesterone Receptor Positive, Stage IB Breast Cancer AJCC v7, Stage IIA Breast Cancer AJCC v6 and v7, Stage IIB Breast Cancer AJCC v6 and v7, Stage IIIA Breast Cancer AJCC v7, Stage IIIB Breast Cancer AJCC v7, Stage IIIC Breast Cancer AJCC v7 | Dana-Farber Cancer Institute | February 21, 2013 |
| NCT02827877 | Recruiting | Breast Cancer | City of Hope Medical Center | July 11, 2016 |
| NCT02514681 | Recruiting | Breast Cancer | Japan Breast Cancer Research Group | August 4, 2015 |
| NCT01597414 | Active, not recruiting | Breast Cancer | European Organisation for Research and Treatment of Cancer - EORTC | May 14, 2012 |
| NCT02320435 | Recruiting | Breast Cancer | Hoffmann-La Roche | December 19, 2014 |
| NCT03493854 | Recruiting | Metastatic Breast Cancer | Hoffmann-La Roche | April 11, 2018 |
| NCT02896855 | Active, not recruiting | Breast Cancer | Hoffmann-La Roche | September 12, 2016 |
| NCT01572038 | Active, not recruiting | Breast Cancer | Hoffmann-La Roche | April 5, 2012 |
| NCT01912963 | Active, not recruiting | Breast Cancer | Dana-Farber Cancer Institute | July 31, 2013 |
| NCT01774786 | Active, not recruiting | Breast Cancer, Pregnancy | Hoffmann-La Roche | January 24, 2013 |
| NCT01358877 | Active, not recruiting | Neoplasms | Hoffmann-La Roche | May 24, 2011 |
| NCT02003209 | Active, not recruiting | Breast Cancer | National Cancer Institute (NCI) | December 6, 2013 |
| NCT02586025 | Active, not recruiting | Metastatic Breast Cancer | Hoffmann-La Roche | October 26, 2015 |
| NCT01491737 | Active, not recruiting | Invasive Breast Cancer | Hoffmann-La Roche | December 14, 2011 |
| NCT01966471 | Active, not recruiting | Breast Cancer, Breast Cancer, Male, Breast Cancer Female, HER2-positive Breast Cancer | Hoffmann-La Roche | October 21, 2013 |
| NCT00567190 | Active, not recruiting | HER2 Positive Breast Cancer, Cardiovascular Abnormalities | Genentech, Inc. | December 4, 2007 |
| NCT02266173 | Recruiting | Locally Advanced Breast Cancer | Hoffmann-La Roche | October 16, 2014 |
| NCT01777958 | Active, not recruiting | Breast Cancer | Hoffmann-La Roche | January 29, 2013 |
| NCT02132949 | Active, not recruiting | Breast Cancer | Hoffmann-La Roche | May 7, 2014 |
| NCT00833963 | Recruiting | Malignant Solid Tumour, Breast Cancer, Malignant Tumor of Colon, GIST, Ovarian Cancer | Genentech, Inc. | February 2, 2009 |
| NCT02091141 | Recruiting | HER2-Positive Metastatic Breast Cancer, HER2-Negative Metastatic Breast Cancer, Locally Advanced or Early Breast Cancer | Genentech, Inc. | March 19, 2014 |
| NCT03112590 | Recruiting | Breast Cancer | H. Lee Moffitt Cancer Center and Research Institute | April 13, 2017 |
| NCT03329378 | Not yet recruiting | Breast Cancer | Icahn School of Medicine at Mount Sinai | November 6, 2017 |
| NCT02605915 | Active, not recruiting | HER2-positive Breast Cancer | Hoffmann-La Roche | November 16, 2015 |
| NCT02402712 | Active, not recruiting | Breast Cancer Metastases, HER2 Positive Breast | Hoffmann-La Roche | March 30, 2015 |
| NCT02286843 | Recruiting | Breast Cancer | Memorial Sloan Kettering Cancer Center | November 10, 2014 |
Table 2. Approved Drugs of Pertuzumab**
| INN (trade name) | Therapeutic area | Dose | Strength | Route | Company | Marketing start | Market |
| Perjeta | HER2-positive Breast Cancer | Concentrate for solution | 30 mg / mL | Intravenous infusion | Genentech | June 8, 2012 |

|
| Perjeta | Breast Neoplasms | Concentrate for solution | 30 mg / mL | Intravenous infusion | Roche Registration GmbH | March 4, 2013 |

|
| Perjeta | HER2-positive Breast Cancer | Concentrate for solution | 30 mg / mL | Intravenous infusion | Hoffmann-La Roche Limited | May 8, 2013 |

|
| Perjeta | HER2-positive Breast Cancer | Concentrate for solution | 30 mg / mL | Intravenous infusion | Roche Products Pty Ltd | May 6, 2013 |

|
| Perjeta | HER2-positive Breast Cancer | Concentrate for solution | 30 mg / mL | Intravenous infusion | Chugai Pharmaceutical Co., Ltd | June 28, 2013 |

|
What We Provide
Therapeutic Antibody
Pertuzumab
We provide high-quality Pertuzumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Pertuzumab
** Information presented in the table were collected from the following websites:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=125409
https://www.ema.europa.eu/medicines/human/EPAR/perjeta
http://search.tga.gov.au/s/search.html?collection=tga-artg&profile=record&meta_i=196218
https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=88984
http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/4291424A1
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

