

Rozanolixizumab Overview
Introduction of Rozanolixizumab
Rozanolixizumab (also known as UCB7665) is an investigational humanized monoclonal IgG antibody for the treatment of myasthenia gravis (MG), a neuromuscular condition thought to be triggered by an autoimmune response. In addition to MG, Rozanolixizumab is also being tested as a potential therapy for patients with idiopathic thrombocytopenic purpura, also known as immune thrombocytopenia (ITP). This drug is developed by UCB S.A., Belgium. The safety, tolerability, pharmacokinetics (movement in the body), and pharmacodynamics (effect on the body) of Rozanolixizumab were evaluated in a placebo-controlled Phase 1 clinical trial (NCT02220153). Preliminary results from the trial presented at the 2017 Peripheral Nerve Society Annual Meeting, showed that Rozanolixizumab effectively reduced serum IgG, with under-the-skin administration generally being better tolerated than injection into the bloodstream. To further these observations, a Phase 2, multi-center, randomized, double-blind, placebo-controlled study (NCT03052751) was initiated to test the effectiveness, safety, and tolerability of Rozanolixizumab in patients with moderate-to-severe MG. The study is expected to be completed by 2018. Rozanolixizumab also is being tested in a Phase 2 trial (NCT02718716) for the treatment of immune thrombocytopenia. Interim results from the trial also confirmed the efficacy of Rozanolixizumab in the reductions of IgG levels.
Mechanism of Action of Rozanolixizumab
Although originally referred to as the neonatal Fc receptor, FcRn influences IgG serum levels and tissue distribution at all stages of life. This divergent member of the MHC class I family molecule is a heterodimer composed of an evolutionally distinct α-chain in complex with the β2-microglobulin (β2M) light chain that is common to most other class I molecules. FcRn is an intracellular trafficking, integral membrane Fc receptor for IgG. It resides primarily in the early acidic endosomes where it captures endocytosed IgG by binding to the Fc region only at a low pH. FcRn rescues bound IgG from degradation in the lysosomal compartment and transports its ligand to the cell surface for release at neutral extracellular pH. Through this mechanism, FcRn is responsible for the long serum half-life of IgG. FcRn transports IgG across the tight epithelial barriers of the endothelium and mucosa, thus influencing its bioavailability. In antigen presenting cells, FcRn controls the presentation of antigens in IgG immune complexes to T cells and IgG-Fc fusion protein vaccines. FcRn also controls serum albumin homeostasis by an analogous trafficking mechanism as for IgG. These properties have proven to be critical for maximizing the pharmacokinetics immunofluorescence and bioavailability of IgG monoclonal antibodies (mAbs) and IgG-Fc fusion proteins and albumin conjugated protein therapeutics. Rozanolixizumab, a humanized high-affinity anti-human neonatal Fc receptor (FcRn) monoclonal antibody, has been developed to reduce pathogenic IgG in autoimmune and alloimmune diseases. High concentrations of pathogenic IgG autoantibodies in the circulation occur due to the action of the neonatal Fc receptor (FcRn) that bind to IgG, inhibiting its degradation. It is believed to compete with FcRn binding, thus resulting in accelerated degradation of endogenous pathogenic IgG antibodies left unbound to FcRn. Rozanolixizumab works by binding with high-affinity to human neonatal Fc receptor (FcRn), selectively inhibiting IgG rescue and recycling. By reducing the serum levels of IgG, it is expected that Rozanolixizumab will help reduce the symptoms of MG.
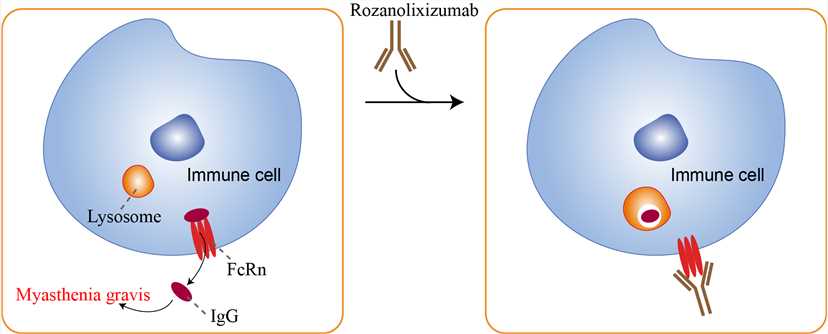
Fig.1 Mechanism of action of Rozanolixizumab
What We Provide
Therapeutic Antibody
Rozanolixizumab
We provide high-quality Rozanolixizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

