

Tabalumab Overview
Introduction of Tabalumab
Tabalumab is a human immunoglobulin G subclass 4 (IgG4)-variant monoclonal antibody (mAb) that binds and neutralizes both membrane and soluble forms of B-cell activating factor (BAFF), which is also known as B lymphocyte stimulator (BLyS) and tumor necrosis factor ligand superfamily member 13B. It was developed by Eli Lilly and Company. The initial phase 1 study of tabalumab included patients both with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), as it was felt that tabalumab could be effective in both populations. The development plan included additional studies in RA and SLE. Based on the results from phase 2 studies in RA patients, phase 3 studies were initiated to establish the efficacy and safety of tabalumab in patients with RA. However, these studies did not demonstrate sufficient efficacy to warrant continued development in RA. The compound is currently in phase 3 testing in SLE.
Mechanism of Action of Tabalumab
Systemic lupus erythematosus (SLE) is considered the prototypic autoimmune disease, with aberrations throughout the immune system resulting in diverse clinical manifestations. B cells are central in the pathogenesis of SLE, because they are precursors for plasma cells that secrete antibodies; they also present antigens to T cells and other B cells, and they secrete proinflammatory cytokines. B cells are activated by T cells. B cells and plasmacytoid dendritic cells can also be activated through endosomal toll-like receptors, some of which recognize DNA- and RNA-containing proteins. In patients with SLE, numbers of circulating plasma cells, plasma blasts, and late transitional B cells (a late stage of maturation in B cells) are increased. Higher-than-normal proportions of B cells and T cells are activated, and activation pathways are abnormal, favoring autoimmunity and inflammation. Regulatory cells of several types that suppress B cells and helper T cells are low in numbers or dysfunctional in most patients with SLE. Increased B-cell activation is due in part to increased levels of growth factors, including BAFF. BAFF is a member of the tumor necrosis factor (TNF) superfamily and is expressed by and cleaved from monocytes, macrophages, dendritic cells, neutrophils, T-cells, stromal cells, astrocytes, fibroblast-like synovial cells, nurse-like cells, osteoclasts, and ductal epithelial cells. It is a transmembrane protein that is also secreted in soluble form and is a principal growth factor for survival during B-cell maturation and development. BAFF receptors (BR3, transmembrane activator and calcium-modulator and cyclophilin ligand interactor [TACI], and B-cell maturation antigen [BCMA]) are present on different immune cells including B-cell and T-cell subsets, and plasma cells. Receptor engagement by BAFF leads to multiple immune cell functional activities including effects on peripheral B-cell survival and maturation, promotion of isotype switching to IgA, and co-stimulatory activity in T-cell activation; all are required in T-independent antibody responses. Tabalumab binds primarily to circulating soluble BAFF thus inhibiting the downstream signaling pathways in the pathologies of SLE.
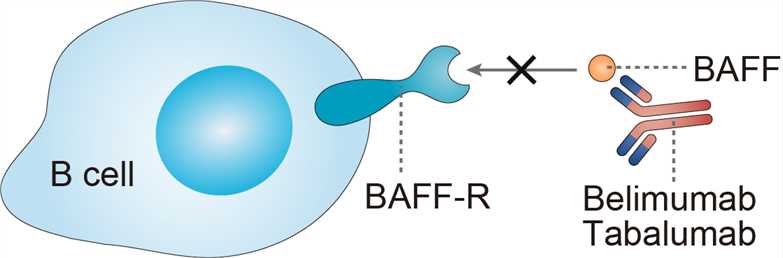
Fig 1. Mechanism of Action of Tabalumab
What We Provide
Therapeutic Antibody
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

