

Vadastuximab Talirine Overview
Introduction of Vadastuximab Talirine
Vadastuximab talirine (SGN-CD33A) is an antibody-drug conjugate (ADC) directed to CD33 (SIGLEC-3), which is a transmembrane receptor expressed on cells of myeloid lineage. The trial drug, being developed by Seattle Genetics and currently in clinical trials, is designed for the treatment of acute myeloid leukemia (AML). SGN-CD33A uses h2H12ec as the CD33 antibody. h2H12ec is a humanized IgG1 antibody derived from a murine clone (m2H12; initially raised against the extracellular portion of CD33) that is engineered to contain a cysteine at position 239 of the heavy chain (Ser239Cys) for site-directed drug conjugation. A highly potent, synthetic DNA cross-linking pyrrolobenzodiazepine (PBD) dimer (SGD-1882) is then conjugated to h2H12ec via a protease-cleavable maleimidocaproyl-valine-alanine linker. PBDs are a class of natural products produced by various actinomycetes, are sequence selective DNA alkylating compounds with significant antitumor properties. As a class of DNA-crosslinking agents, they are significantly more potent than systemic chemotherapeutic drugs. Some PBDs have the ability to recognize and bond to specific sequences of DNA. With antibody engineering, very homogenous drug loading is accomplished, averaging 1.9 PBD moieties per antibody. In preclinical studies, SGN-CD33A was more potent than gemtuzumab ozogamicin (GO) against a panel of human AML cell lines and a series of primary AML samples in vitro as well as in immunodeficient mice in vivo. Unlike GO, SGN-CD33A maintained anti-leukemia activity in the presence of drug transporters in preclinical AML models. Although the drug’s anti-leukemia efficacy is now well established, concerns over toxicity to the liver and normal hematopoietic cells have derailed the clinical development of SGN-CD33A, and its future is uncertain. The first concern dates back to late December 2016, when the manufacturer (Seattle Genetics, Inc.; Bothell, WA, USA) announced that the Food and Drug Administration (FDA) had placed a hold or partial hold on several of the trials with SGN-CD33A to further evaluate the potential risk of drug-induced hepatotoxicity when administered before or after allogeneic hematopoietic cell transplantation (HCT). These holds arose from the identification of six patients experiencing hepatotoxicity, including several cases of sinusoidal obstruction syndrome (SOS), with four resulting deaths. While medical details of these cases have so far not been released, enrollment on the phase 1/2 trial testing SGN-CD33A before and after allogeneic HCT was permanently discontinued. In June 2017, patient enrollment and treatment in all ongoing trials of SGN-CD33A was suspended and the CASCADE trial permanently discontinued because of a higher rate of deaths, including fatal infections, in the SGN-CD33A-containing arm of the CASCADE trial.
Mechanism of Action of Vadastuximab Talirine
The CD33 antigen is a sialic acid-dependent adhesion protein that is specific for myeloid cells. CD33 is expressed in approximately 90% of AML cases, as defined by the presence of the antigen on > 20% of the leukemic blasts but not on normal CD34+ pluripotent hematopoietic stem cells or non-hematopoietic tissues. Vadastuximab talirine targets CD33-expressing cells. Binding to the antigen is followed by endocytosis. The covalent link is cleaved inside lysosomes, allowing PBD release. PBD dimers bind in the minor groove of DNA, where they form covalent aminol crosslinks between the N2 of guanine and the C11 position of the PBD. The resulting PBD-DNA adducts cause replication forks to stall and tumor cells to arrest at the G2-M boundary, ultimately resulting in apoptosis at low nanomolar to picomolar concentrations.
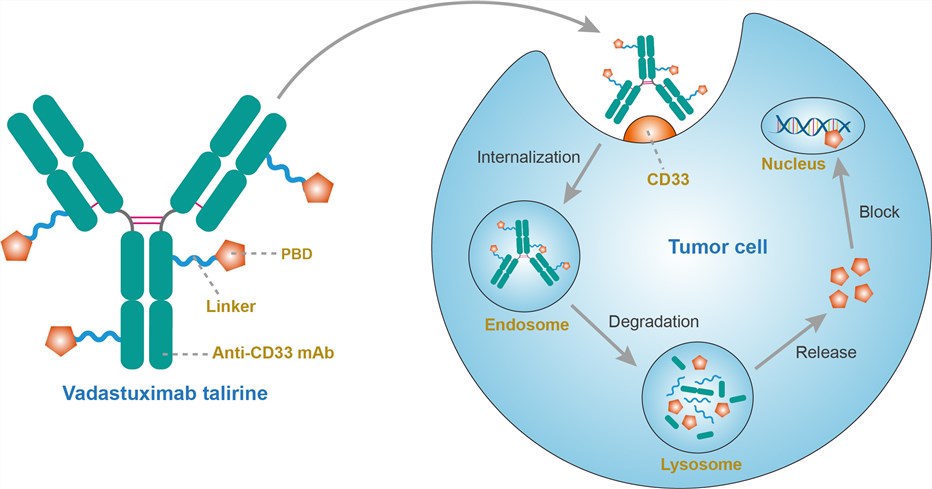 Fig.1 Mechanism of action of vadastuximab talirine
Fig.1 Mechanism of action of vadastuximab talirine
What We Provide
Therapeutic Antibody
Vadastuximab Talirine
We provide high-quality Vadastuximab Talirine for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

