

Cixutumumab Overview
Introduction of Cixutumumab
Cixutumumab (IMC-A12) is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor (IGF-1R) with potential antineoplastic activity for the treatment of solid tumors. This drug was developed by ImClone Systems, since acquired by Eli Lilly, using phage display technology from Dyax. Cixutumumab was well tolerated when used alone and in combination therapies, but its antitumor activity was low in the existing phase II clinical trials. An acceptable incidence of adverse effects supports further investigation of this drug, provided that it shows antitumor activity in combination with other drugs. Now, cixutumumab is involved in two cancer clinical trials, including esophageal cancer and breast cancer.
Mechanism of Action of Cixutumumab
The insulin family of growth factors is an evolutionally conserved system which plays a crucial role in the growth and development of many tissues and the regulation of overall growth and metabolism. This system comprises three receptors [insulin receptor (IR), IGF-1 receptor (IGF-1R), and IGF-2/mannose 6-phosphate receptor (M-6-PR)], three ligands (insulin, IGF-1, and IGF-2), and six known types of circulating IGF-binding proteins (IGFBP1-6). The IGF-1R is highly homologous to the IR.
The IGF-1R is a receptor tyrosine kinase with a structure of a heterotetrameric glycoprotein composed of two α and two β subunits, post-transcriptionally linked by disulfide bonds, which regulates cell survival and cell cycle progression via the PI3K/Akt and extracellular signal regulated kinase pathways through insulin receptor substrate-1to-4 (IRS-1to-4) and Src-homology collagen (Shc) adapter proteins. Phosphorylation of the IRS adapter molecules on one hand triggers activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, whereas, on the other hand, the Shc adapter activates signaling by the Ras/Raf/MEK/Erk signaling pathway. IGF-IR seems to be expressed in most human cancers. The two IGF-1R ligands, IGF-1 and IGF-2, have been implicated in cancer initiation and progression.
In the past decades, a large amount of evidence has emphasized that IGF-IR play a key role in the transformation of cells, cancer cell proliferation, as well as in metastatic events, associated in various types of human cancers. It seems that inhibition of IGF-IR signaling can generate antineoplastic effects. A number of monoclonal antibodies have been developed to target the receptor itself, which bind to the extracellular domains of the IGF-1R and block ligand binding. A feature common to all anti-IGF-1R antibodies, probably more important than the blocking activity itself, is their ability to down-regulate of the IGF-1R overtime by promoting internalization of the receptor. Receptor targeting antibodies might have important therapeutic advantages, concerning both specificity and toxicity. A variety of fully human anti-IGF-1R monoclonal antibodies have been characterized and showed strong anti-tumor activity in vitro and in vivo.
Cixutumumab is a fully human immunoglobulin G1 (IgG1) monoclonal antibody that specifically inhibits IGF-1R signaling. Binding cixutumumab to IGF-1R results in receptor internalization and degradation. Because cixutumumab is an IgG1 monoclonal antibody, it may induce additional cytotoxicity via immune effector mechanisms such as antibody-dependent cellular cytotoxicity. In preclinical studies, cixutumumab monotherapy resulted in growth inhibition of multiple experimental cancers. Moreover, cixutumumab safely enhanced the tumor growth inhibitory and cytotoxic effects of a broad range of chemotherapeutics, and modulated the action of agents that target hormone receptors and signal transduction, which may have implications for cancer therapy.
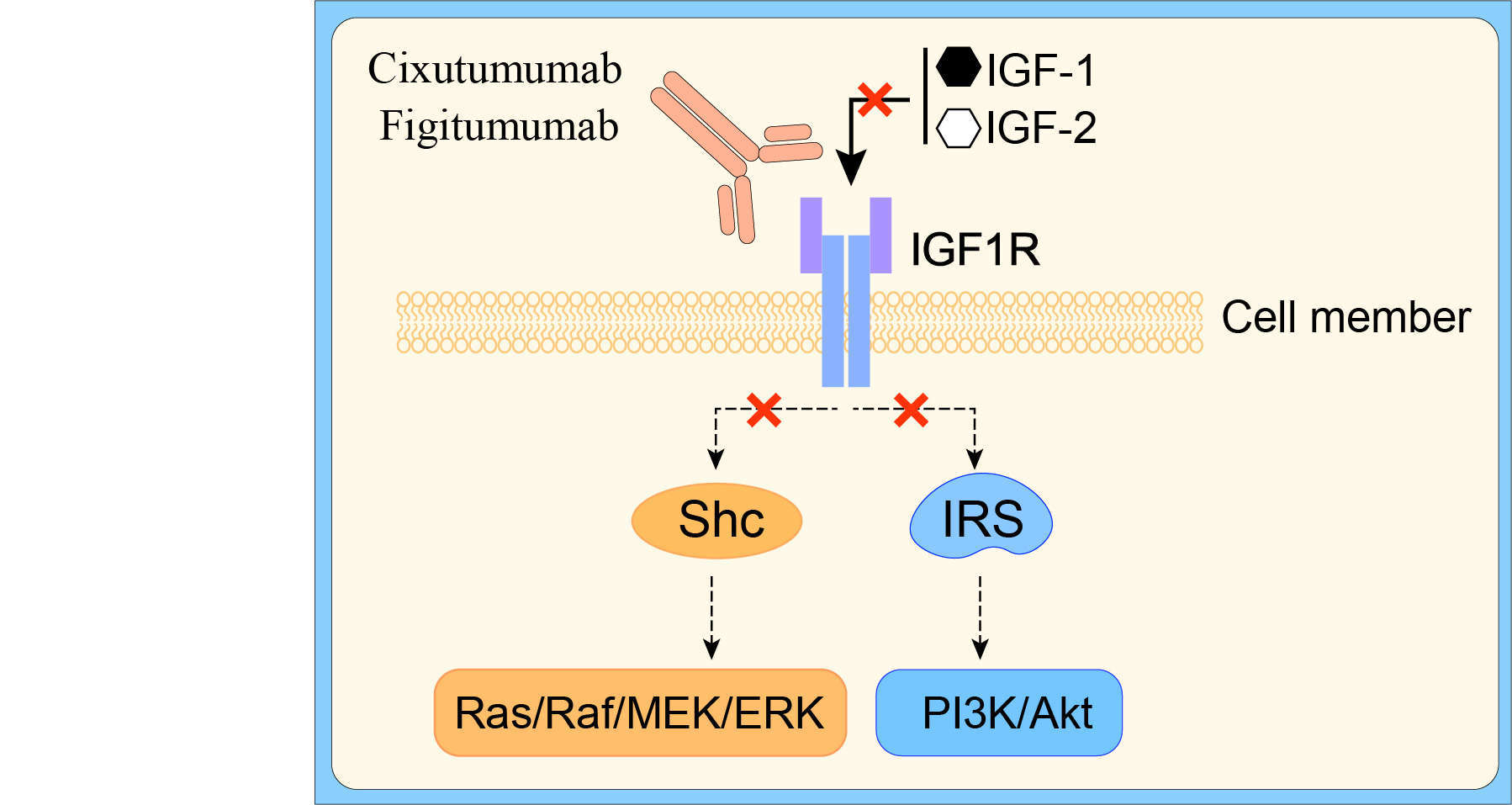 Figure 1 Mechanism of Action of Cixutumumab
Figure 1 Mechanism of Action of Cixutumumab
Clinical Projects of Cixutumumab *
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT01142388 | Active, not recruiting | Esophageal Adenocarcinoma, Esophageal Squamous Cell Carcinoma, Gastroesophageal Junction Adenocarcinoma, Recurrent Esophageal Carcinoma, Stage IV Esophageal Cancer AJCC v7 | National Cancer Institute (NCI) | June 5, 2018 |
| NCT00684983 | Active, not recruiting | HER2/Neu Positive, Recurrent Breast Carcinoma, Stage IIIB Breast Cancer AJCC v7, Stage IIIC Breast Cancer AJCC v7, Stage IV Breast Cancer AJCC v6 and v7 | National Cancer Institute (NCI) | July 2, 2018 |
What We Provide
Therapeutic Antibody
Cixutumumab
We provide high-quality Cixutumumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?term=Cixutumumab&Search=Apply&recrs=b&recrs=a&recrs=f&recrs=d&age_v=&gndr=&type=&rslt=
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

