

Demcizumab Overview
Introduction of Demcizumab
Demcizumab (as known as OMP-21M18) is a humanized monoclonal antibody directed against the N-terminal epitope of Notch ligand DLL4 (delta-like 4) with potential antineoplastic activity. Demcizumab was developed by OncoMed Pharmaceuticals in collaboration with Celgene. Demcizumab is firstly used to treat patients with pancreatic cancer or non-small cell lung cancer. In preclinical studies demcizumab demonstrated the ability to block Notch signaling and inhibit tumor growth through multiple mechanisms, such as the reduction of cancer stem cell frequency and the inhibition of angiogenesis. Phase I data demonstrated that demcizumab was generally well tolerated and had promising antitumor activity in patients with advanced solid tumors. This anti-DLL4 MAb was used to be tested in additional phase I and phase II studies in combination with gemcitabine with or without nabpaclitaxel in pancreatic cancer, with FOLFIRI in colorectal cancer, with carboplatin and pemetrexed in lung cancer and with paclitaxel in patients with platinum-resistant ovarian cancer. However, Phase II combination therapy study in front-line nonsquamous, non-small-cell lung cancer (NSCLC) and Phase II PINNACLE study evaluating the anti-Notch2/3 candidate tarextumab as combination therapy in small-cell lung cancer both failed to meet its primary endpoints. Simultaneously, demcizumab has been known to cause many adverse effects in patient. The most common side effects are hypertension, fatigue, anemia, and headaches. More adverse effect are nausea, hypoalbuminemia, dizziness, dyspnea, and heart related illness occurred.
Mechanism of Action of Demcizumab
Tumor angiogenesis is a prerequisite for tumor growth to provide adequate acquisition of blood supply and is implicated in the development of perilesional edema by increased levels of angiogenic factors and tumor vessels with high permeability, one signaling pathway contributing to this process is Notch pathway. Delta-like ligand 4 (DLL4) is 1 of the known transmembranous Notch ligands and is expressed at the site of vascular development and angiogenesis. Physiologically, upregulated DLL4 activation by vascular endothelial growth factor (VEGF) and hypoxia triggers a negative feedback that inhibits the superfluous VEGF effect of vascular sprouting. But in tumor angiogenesis, DLL4 induces larger tumor vessel to enhance the vascular structure and function. Accumulating evidence shows that the DLL4-Notch pathway can enhance the activity of VEGF on tumor cells, promote its expression, and stimulate the formation of tumor angiogenesis to promote tumor growth/invasion. Noticeably, DLL4 expression has been proven to be correlated with poor prognosis in breast cancer, nasopharyngeal carcinoma, and colon cancer. Demcizumab is a humanized monoclonal antibody targeting DLL4, and it binds to the membrane-binding portion of DLL4 and prevents its interaction with Notch-1 and Notch-4 receptors, thereby inhibiting Notch-mediated signaling and gene transcription, which may impede tumor angiogenesis. Based on preclinical studies, demcizumab may have a multi-pronged mechanism of action: halting cancer stem cell growth and reducing cancer stem cell frequency, disrupting angiogenesis in the tumor and augmenting anti-tumor immune responses by decreasing tumor myeloid-derived suppressor cells (MDSCs).
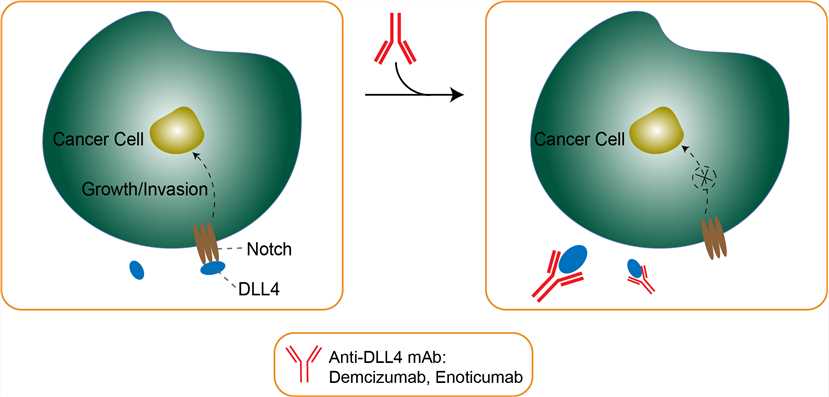
Fig 1. Mechanism of Action of Demcizumab
What We Provide
Therapeutic Antibody
Demcizumab
We provide high-quality Demcizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

