

Muromonab-CD3 Overview
Introduction of Muromonab-CD3
Muromonab-CD3, trade name Orthoclone OKT3, is a recombinant murine (mouse) monoclonal antibody to human CD3. More specifically it is 93% monomeric immune globulin G type 2a (IgG2a) and directed against the CD3 (T3) receptor on the surface of human T-cells (T-lymphocytes) cultured using the murine ascites method. Muromonab-CD3 was approved for use in treating acute rejection after renal transplantation in 1997 which was the first monoclonal antibody to be approved for clinical use in humans and its indications were later broadened to include rejection after heart and liver transplantation. Under the brand name Orthoclone OKT3, this drug is available in liquid solution in 5 mL ampules of 5 mg. However, it is a potent immunosuppressive agent and may result in reactivation of hepatitis B in susceptible patients. Furthermore, the initial engagement of CD3 receptors can result in a transient, acute release of proinflammatory cytokines (cytokine release syndrome) with symptoms of high fever, weakness, dyspnea, nausea, chest pain and diarrhea arising within the first two days of starting therapy.
Mechanism of Action of Muromonab-CD3
Transplantation of solid organs has emerged as a viable therapeutic modality for the treatment of a variety of ailments, such as end stage renal disease. Rejection of solid organ allografts is the result of a complex series of interactions involving coordination between both the innate and adaptive immune system with T cells central to this process. The ability of recipient T cells to recognize donor-derived antigens, called allorecognition, initiates allograft rejection. Once recipient T cells become activated, they undergo clonal expansion, differentiate into effector cells, and migrate into the graft where they promote tissue destruction. Muromonab-CD3 (OKT3) is the first anti-CD3 monoclonal antibody (mAb) available for treatment in humans. It is a murine mAb of the IgG-2a class, directed against the CD3 molecule which is present on the surface of human thymocytes and mature T cells. The CD3 molecule is closely associated with the T cell antigen receptor (TCR) in the so-called CD3/TCR complex. This complex plays a vital role in T cell function: antigen recognition by the TCR results in transmembrane signal transduction via the CD3 molecule and subsequent T cell proliferation and activation of cytotoxic cells. Muromonab-CD3 binds to the T cell receptor-CD3-complex (specifically the CD3 epsilon chain) on the surface of circulating T cells, initially leading to an activation, but subsequently inducing blockage and apoptosis of the T cells. This protects the transplant against the T cells. It also has some adverse effects. Especially during the first infusion, the binding of muromonab-CD3 to CD3 can activate T cells to release cytokines like tumor necrosis factor and interferon gamma. This cytokine release syndrome, or CRS, includes side effects like skin reactions, fatigue, fever, chills, myalgia, headaches, nausea and diarrhea.
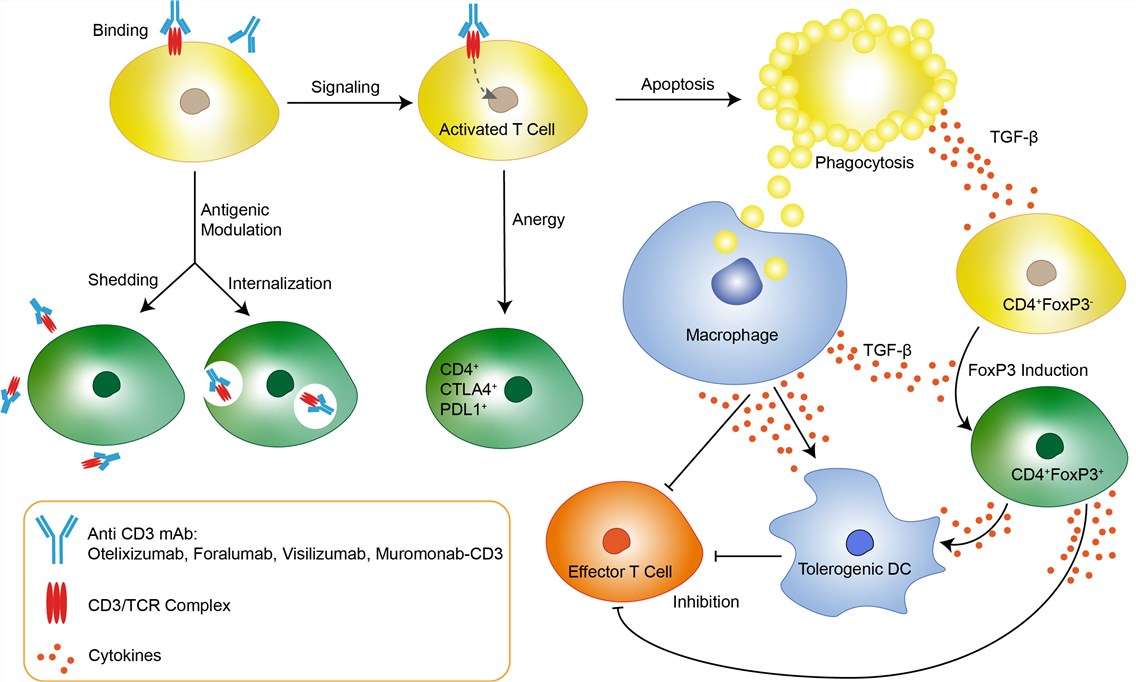 Fig 1. Mechanism of Action of Muromonab-CD3
Fig 1. Mechanism of Action of Muromonab-CD3
Table 1. Clinical Projects of Muromonab-CD3*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT03291249 | Not yet recruiting | NASH - Nonalcoholic Steatohepatitis, NAFLD, T2DM (Type 2 Diabetes Mellitus) | Tiziana Life Sciences, PLC | September 25, 2017 |
| NCT01420874 | Active, not recruiting | Colorectal Cancer, Cancer of Pancreas, Pancreatic Neoplasm, Malignant Neoplasm of Large Intestine, Malignant Tumor of Colon, Colon Carcinoma, Cancer of Colon, Pancreatic Cancer | Barbara Ann Karmanos Cancer Institute | August 22, 2011 |
| NCT02620865 | Active, not recruiting | Metastatic Pancreatic Adenocarcinoma, Recurrent Pancreatic Carcinoma, Stage III Pancreatic Cancer, Stage IV Pancreatic Cancer | Barbara Ann Karmanos Cancer Institute | December 3, 2015 |
| NCT01022138 | Active, not recruiting | Breast Cancer | Barbara Ann Karmanos Cancer Institute | December 1, 2009 |
| NCT03406858 | Recruiting | Castration Levels of Testosterone, Castration-Resistant Prostate Carcinoma, Prostate Carcinoma Metastatic in the Bone, PSA Progression, Stage IV Prostate Adenocarcinoma AJCC v7 | Barbara Ann Karmanos Cancer Institute | January 23, 2018 |
| NCT03269526 | Recruiting | Locally Advanced Pancreatic Adenocarcinoma, Metastatic Pancreatic Adenocarcinoma | University of Virginia | August 31, 2017 |
| NCT01030861 | Active, not recruiting | Autoantibody Positive, Non-diabetic Relatives at Risk for Type 1 Diabetes, High Risk, Impaired Glucose Tolerance | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) | December 14, 2009 |
| NCT03146637 | Recruiting | Advanced Liver Cancer | Benhealth Biopharmaceutical (Shenzhen) Co., Ltd. | May 10, 2017 |
| NCT03540199 | Recruiting | Advanced Kidney Cancer | Fuda Cancer Hospital, Guangzhou | May 30, 2018 |
| NCT03554395 | Recruiting | Advanced Gastric Cancer | Fuda Cancer Hospital, Guangzhou | June 13, 2018 |
| NCT03501056 | Recruiting | Advanced Lung Cancer | Fuda Cancer Hospital, Guangzhou | April 17, 2018 |
| NCT03524261 | Recruiting | Advanced Breast Cancer | Fuda Cancer Hospital, Guangzhou | May 14, 2018 |
| NCT03484962 | Recruiting | Advanced Liver Cancer | Fuda Cancer Hospital, Guangzhou | April 2, 2018 |
| NCT03524274 | Recruiting | Advanced Colorectal Cancer | Fuda Cancer Hospital, Guangzhou | May 14, 2018 |
| NCT03509298 | Recruiting | Advanced Pancreatic Cancer | Fuda Cancer Hospital, Guangzhou | April 26, 2018 |
| NCT00566696 | Active, not recruiting | Leukemia, Acute Lymphocytic (ALL), Leukemia, Myeloid, Acute(AML), Leukemia, Myeloid, Chronic(CML), Juvenile Myelomonocytic Leukemia (JMML), Hemoglobinuria, Paroxysmal Nocturnal (PNH) Hodgkin Lymphoma, Lymphoma, Non-Hodgkin (NHL), Myelodysplastic Syndrome (MDS) | St. Jude Children's Research Hospital | December 3, 2007 |
| NCT03661424 | Not yet recruiting | Breast Cancer Female, Leptomeningeal Metastases | University of Virginia | September 7, 2018 |
| NCT03272334 | Recruiting | Metastatic Breast Cancer | University of Virginia | September 5, 2017 |
| NCT03344250 | Recruiting | Glioblastoma, Glioblastoma Multiforme | University of Virginia | November 17, 2017 |
| NCT02173093 | Recruiting | Desmoplastic Small Round Cell Tumor, Disseminated Neuroblastoma, Metastatic Osteosarcoma, Recurrent Neuroblastoma, Recurrent Osteosarcoma | Barbara Ann Karmanos Cancer Institute | June 24, 2014 |
| NCT03515551 | Recruiting | Melanoma, Advanced NSCLC, Urothelial Carcinoma, Synovial Sarcoma | Immunocore Ltd | May 3, 2018 |
| NCT02651662 | Recruiting | Lymphoma | Regeneron Pharmaceuticals | January 11, 2016 |
| NCT02290951 | Recruiting | Non-Hodgkin's Lymphoma, Chronic Lymphocytic Leukemia | Regeneron Pharmaceuticals | November 14, 2014 |
| NCT01147016 | Active, not recruiting | Breast Cancer | Barbara Ann Karmanos Cancer Institute | June 22, 2010 |
| NCT02342613 | Active, not recruiting | Hematologic Malignancies, Graft-Versus-Host Disease | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | January 21, 2015 |
| NCT01883297 | Recruiting | Recurrent, Platinum Resistant High Grade Serous Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | University Health Network, Toronto | June 21, 2013 |
| NCT03291639 | Not yet recruiting | IL-17+ CD8 T Cells in Cancer Patients | China Medical University Hospital | September 25, 2017 |
| NCT03173430 | Recruiting | Multiple Myeloma | Abramson Cancer Center of the University of Pennsylvania | June 1, 2017 |
| NCT02889861 | Recruiting | Malignant Melanoma | Immunocore Ltd | September 7, 2016 |
| NCT02570308 | Recruiting | Uveal Melanoma | Immunocore Ltd | October 7, 2015 |
| NCT02497898 | Not yet recruiting | Lymphoma, Non-Hodgkin | The First People's Hospital of Changzhou | July 15, 2015 |
| NCT02997761 | Recruiting | Adult B Acute Lymphoblastic Leukemia, Philadelphia Chromosome Positive | University of California, Davis | December 20, 2016 |
| NCT03512405 | Not yet recruiting | CD19 Positive, Philadelphia Chromosome Positive, Recurrent Acute Lymphoblastic Leukemia, Refractory Acute Lymphoblastic Leukemia | City of Hope Medical Center | April 30, 2018 |
| NCT02568553 | Recruiting | CD19 Positive, Mediastinal Lymphoma, Recurrent B-Cell Lymphoma, Unclassifiable, With Features Intermediate Between Diffuse Large B-Cell Lymphoma and Classic, Hodgkin Lymphoma, Recurrent Burkitt Lymphoma, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Grade 1 Follicular Lymphoma, Recurrent Grade 2 Follicular Lymphoma, Recurrent Grade 3 Follicular Lymphoma, Recurrent Mantle Cell Lymphoma, Recurrent Marginal Zone Lymphoma, Recurrent Mediastinal Lymphoma, Recurrent Non-Hodgkin Lymphoma, Recurrent Small Lymphocytic Lymphoma, Refractory B-Cell Lymphoma, Unclassifiable, With Features Intermediate Between, Diffuse Large B-Cell Lymphoma and Classic Hodgkin Lymphoma, Refractory Burkitt Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Follicular Lymphoma, Refractory Mantle Cell Lymphoma, Refractory Marginal Zone Lymphoma, Refractory Mediastinal Lymphoma, Refractory Non-Hodgkin Lymphoma, Refractory Small Lymphocytic Lymphoma | National Cancer Institute (NCI) | October 6, 2015 |
| NCT01352286 | Active, not recruiting | Multiple Myeloma | Adaptimmune | May 11, 2011 |
| NCT02103387 | Active, not recruiting | Breast Cancer | University of Miami | April 3, 2014 |
| NCT02879695 | Recruiting | B Acute Lymphoblastic Leukemia,B Acute Lymphoblastic Leukemia With t(9;22)(q34.1;q11.2); BCR-ABL1, CD19-Positive Neoplastic Cells Present, Mixed Phenotype Acute Leukemia, Mixed Phenotype Acute Leukemia With t(9;22)(q34.1;q11.2); BCR-ABL1, Recurrent B Acute Lymphoblastic Leukemia, Refractory B Acute Lymphoblastic Leukemia | National Cancer Institute (NCI) | August 26, 2016 |
| NCT03294824 | Recruiting | Allogeneic Hematopoietic Stem Cell Transplantation | Assistance Publique - Hôpitaux de Paris | September 27, 2017 |
| NCT02535078 | Recruiting | Malignant Melanoma | Immunocore Ltd | August 28, 2015 |
| NCT02143414 | Recruiting | Acute Lymphoblastic Leukemia, B Acute Lymphoblastic Leukemia, B Acute Lymphoblastic Leukemia With t(9;22)(q34.1;q11.2); BCR-ABL1, B Acute Lymphoblastic Leukemia, Philadelphia Chromosome Negative, Philadelphia Chromosome Positive, Recurrent Adult Acute Lymphoblastic Leukemia, Refractory Adult Acute Lymphoblastic Leukemia, Untreated Adult Acute Lymphoblastic Leukemia | National Cancer Institute (NCI) | May 21, 2014 |
| NCT03478670 | Recruiting | Immunologic Deficiency Syndromes | GlaxoSmithKline | March 27, 2018 |
| NCT02003222 | Recruiting | Acute Lymphoblastic Leukemia, B Acute Lymphoblastic Leukemia, Philadelphia Chromosome Negative, BCR/ABL1 Fusion Protein Negative, Untreated Adult Acute Lymphoblastic Leukemia | National Cancer Institute (NCI) | December 6, 2013 |
| NCT02101853 | Recruiting | B Acute Lymphoblastic Leukemia | National Cancer Institute (NCI) | April 2, 2014 |
| NCT02765126 | Recruiting | Heterologous Effects of Vaccines | Clifford Craig Medical Research Trust | May 6, 2016 |
| NCT03157284 | Active, not recruiting | Atopic Dermatitis | Heinz Italia SpA | May 17, 2017 |
| NCT02860559 | Not yet recruiting | Severe Combined Immunodeficiency | Taiga Biotechnologies, Inc. | August 9, 2016 |
| NCT03429907 | Recruiting | Malignant Neoplasm of Breast | M.D. Anderson Cancer Center | February 12, 2018 |
| NCT03135769 | Recruiting | Gestational Trophoblastic Neoplasias (GTN) | Hospices Civils de Lyon | May 1, 2017 |
What We Provide
Therapeutic Antibody
>Muromonab-CD3
We provide high-quality Muromonab-CD3 for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Reference
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Muromonab-CD3
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

