

Talizumab Overview
Introduction of Talizumab
Talizumab (as known as TNX-901) is a humanized monoclonal antibody that was under development by Tanox in Houston, Texas as a new-concept therapeutic for allergic diseases. The unique anti-IgE antibody was designed to target immunoglobulin E and IgE-expressing B lymphocytes specifically, without binding to IgE already bound by the high affinity IgE receptors on mast cells and basophils. Talizumab was tested in clinical trials at National Jewish Medical and Research Center and other medical centers and allergy clinics across the U. S. and shown to be able to prevent allergic reactions to accidental exposure to peanuts, which is contained in many kinds of foods. One clinical trial result indicated that with the administration of TNX-901, patients, who could tolerate an average of half a peanut before the treatment, were able to ingest up to 9 peanuts before they started to have allergic reactions. Therefore, TNX-901 cannot cure peanut allergy, but could protect patients from the often violent and life-threatening reactions upon the accidental exposure to peanut. Another anti-IgE antibody omalizumab with identical antigen-binding characteristics is already on the market for allergic asthma. Omalizumab is also an anti-IgE monoclonal antibody, which was placed in a development program under a tripartite partnership formed by Tanox, Novartis, and Genentech in 1996. However, it has not been approved for use in food allergies.
Mechanism of Action of Talizumab
Peanut allergy is characterized by symptoms and signs after ingestion that may include nausea, vomiting, diarrhea, abdominal pain, urticaria, angioedema, bronchospasm, hypotension, loss of consciousness, and death. Although data from animals demonstrate that allergic reactions are mediated by antigen-specific IgE bound to high-affinity receptors for IgE (FcεRIs) on mast cells and basophils, non-IgE pathways for anaphylaxis exist, at least in mice and direct clinical evidence of IgE involvement in peanut allergy in humans is lacking. Approximately 1.5 million people in the United States have peanut allergy 50 to 100 of whom die each year from unintended ingestion. Severe reactions can occur at any age the previous reaction cannot be used reliably to predict the course of the next, and even the first reaction may be severe. Current treatment for peanut allergy is avoidance or rescue with epinephrine. Only a small minority of patients who are allergic to peanuts carry epinephrine, and even timely injection may not prevent death. Avoidance is extremely difficult and the risk–benefit ratio for hyposensitization is unfavorable. Talizumab is a humanized IgG1 monoclonal antibody against IgE that binds with high affinity to an epitope in the CH3 domain, masking a region responsible for binding to both FcεRIs and low-affinity Fcε receptors (FcεRII, or CD23). In addition to inhibiting binding of IgE to mast cells and basophils, anti-IgE also markedly down-regulates the expression of FcεRIs on basophils and may inhibit allergen-specific activation of T cells through interference with the processing of antigen-presenting cells mediated by FcεRIIs or FcεRIs.
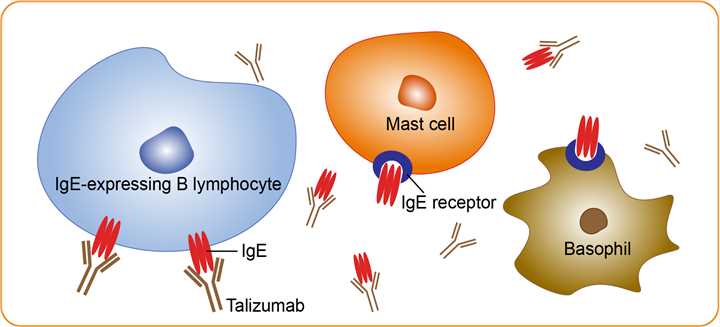
Fig.1 Mechanism of action of Talizumab
What We Provide
Therapeutic Antibody
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

