

Tildrakizumab Overview
Introduction of Tildrakizumab
Tildrakizumab is a high-affinity, humanized, monoclonal IgG1κ antibody targeting the p19 subunit of interleukin (IL)-23, a cytokine that plays an important role in managing the immune system and autoimmune disease. Tildrakizumab is designed by Schering-Plough (now part of Merck's clinical program) for the treatment of immunologically mediated inflammatory disorders. In March 2018, it was approved by the Food and Drug Administration for the treatment of moderate-to-severe plaque psoriasis as an injection for subcutaneous use in the United States.
Mechanism of Action of Tildrakizumab
IL-23 is responsible for the development and differentiation of T-helper 17 (Th17) cells, enhancing the expression of IL-17A and IL-17B along with other pro-inflammatory cytokines that activate keratinocytes and perpetuate the inflammatory cascade, thus IL-23 is recognized as a crucial player in the pathogenesis of chronic autoimmune diseases in general and of psoriasis in particular. Tildrakizumab targets the p19 subunit of IL-23, acting to halt the inflammatory Th17 pathway, thereby behaves the anti-psoriasis efficacy.
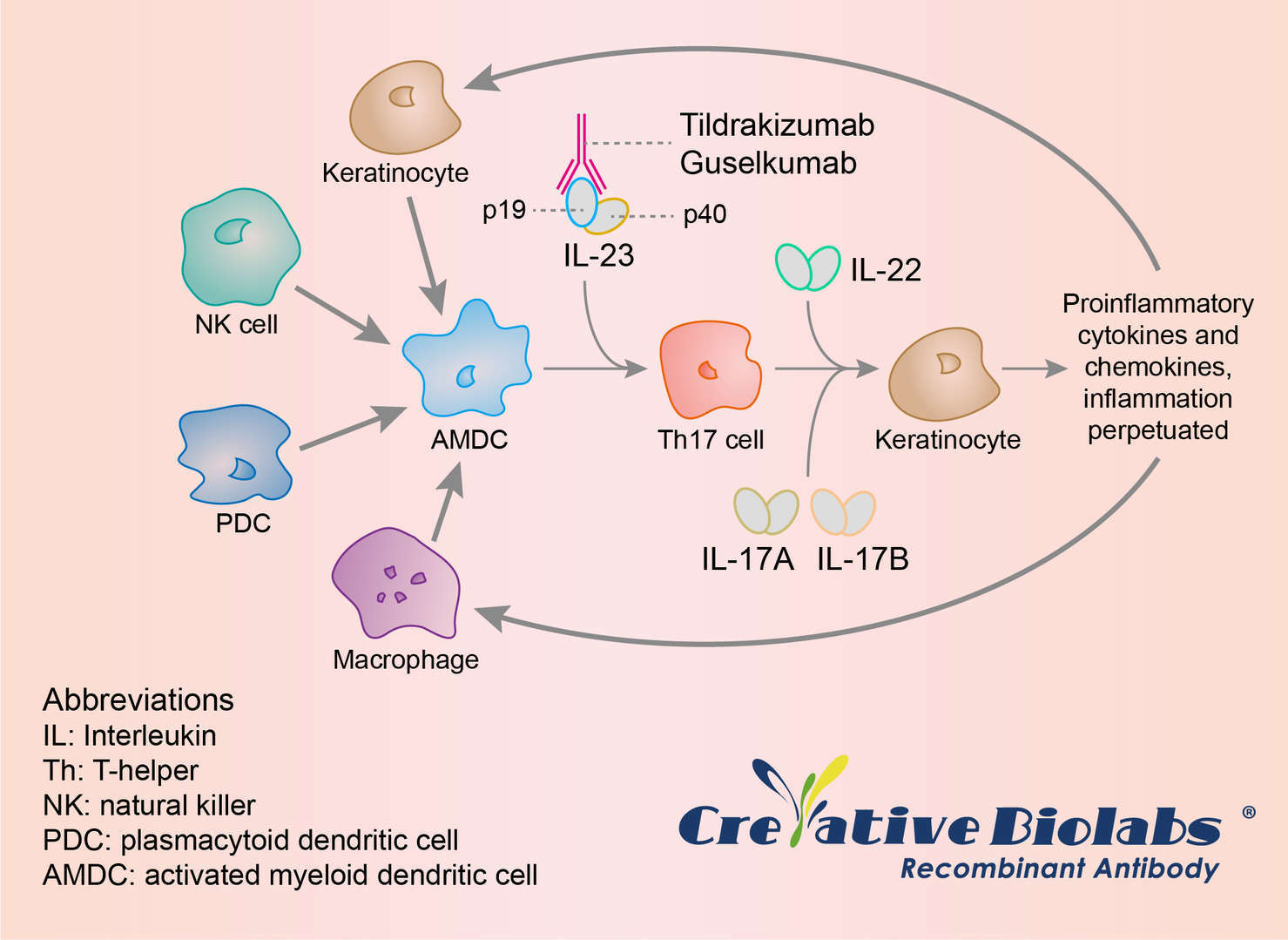 Fig.1 Mechanism of Action of Tildrakizumab
Fig.1 Mechanism of Action of Tildrakizumab
Clinical Projects of Tildrakizumab*
| NCT ID | Status | Conditions | Lead Sponsor | Update Time |
| NCT01729754 | Active, not recruiting | Psoriasis | Merck Sharp & Dohme Corp. | November 20, 2012 |
| NCT02980692 | Recruiting | Psoriasis | Sun Pharma Global FZE | December 2, 2016 |
| NCT02980705 | Recruiting | Psoriasis | Sun Pharma Global FZE | December 2, 2016 |
| NCT01722331 | Active, not recruiting | Psoriasis | Merck Sharp & Dohme Corp. | November 6, 2012 |
Approved Drugs of Tildrakizumab**
| INN (trade name) | Therapeutic area | Dosage | Strength | Route | Company | Marketing start | Market |
| Ilumya | Plaque Psoriasis | Injection | 100 mg/1mL | Subcutaneous | Merck Sharp & Dohme Corp. | March 20, 2018 |

|
What We Provide
Therapeutic Antibody
Tildrakizumab
We provide high-quality Tildrakizumab for use in WB, FC, IP, ELISA, Neut, FuncS, IF and most other immunological methods. For lab research use only, not for diagnostic, therapeutic or any in vivo human use.
Resources
* The table was excerpted from the following website
https://clinicaltrials.gov/ct2/results?cond=&term=Tildrakizumab
** Information presented in the table were collected from the following websites:
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&applno=761067
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

