 Loading...
Loading...

TBK1
Disease related genes, Enzymes, Human disease related genes, Metabolic proteins, Potential drug targets, RAS pathway related proteins
Intracellular
Low cell type specificity
Low immune cell specificity
Low cell line specificity
Homodimer (PubMed:21145761). Interacts with DDX3X, TIRAP and TRAF2 (PubMed:10581243, PubMed:14530355). Part of a ternary complex consisting of TANK, TRAF2 and TBK1 (PubMed:10581243). Interacts with AZI2, TANK and TBKBP1; these interactions are mutually exclusive and mediate TBK1 activation (PubMed:14560022, PubMed:21931631, PubMed:23453972, PubMed:10581243, PubMed:29251827). Interacts with GSK3B; this interaction promotes TBK1 self-association and autophosphorylation (PubMed:21145761). Interacts with SIKE1; SIKE1 is associated with TBK1 under physiological condition and dissociated from TBK1 upon viral infection or TLR3 stimulation (PubMed:16281057). Interacts with IRF3, leading to IRF3 phosphorylation (PubMed:14703513, PubMed:25636800). Interacts with DDX58/RIG-I (PubMed:16281057). Interacts with CYLD (PubMed:18636086, PubMed:32185393). Interacts with OPTN and TRAF3 (PubMed:20174559). Interacts with SRC (PubMed:19419966). Interacts with the exocyst complex subunit SEC5/EXOC2; this interaction is sufficient to trigger TBK1 activity (PubMed:17018283). Interacts with STING1, leading to STING1 phosphorylation (PubMed:19416887, PubMed:25636800, PubMed:30842653). Interacts with IFIT3 (via N-terminus) (PubMed:21813773). Interacts with MAVS; interaction only takes place in the presence of IFIT3 and leads to MAVS phosphorylation (PubMed:21813773, PubMed:25636800, PubMed:28011935). Interacts (via protein kinase domain) with TTLL12 (via TTL domain); the interaction prevents MAVS binding to TBK1 (PubMed:28011935). Interacts with TICAM1; this interaction is enhanced in the presence of WDFY1 and leads to TICAM1 phosphorylation (PubMed:14530355, PubMed:14739303, PubMed:25736436, PubMed:25636800). Interacts with TRIM26 (PubMed:26611359). Interacts with TRIM23 (PubMed:28871090). Interacts with TTC4 and IKBKE (PubMed:29251827). Interacts with HNRNPA2B1 (PubMed:31320558). Interacts with DDX3X (PubMed:20375222). Interacts with TRIM14 (PubMed:32404352). Interacts with CEP170; efficient complex formation may be dependent on the presence of CCDC61 (PubMed:30354798). Interacts with TRAF3IP3 (PubMed:32366851). Interacts with HSP90AA1; the interaction mediates TBK1 association with TOMM70 (PubMed:20628368). (Microbial infection) Interacts with Borna disease virus (BDV) P protein leading to its phosphorylation. (Microbial infection) Interacts with Ebola virus protein VP35. (Microbial infection) Interacts with HCV NS3; this interaction leads to inhibition of cellular antiviral response by blocking necessary interactions between the TBK1 and its substrates IRF3 and IRF7. (Microbial infection) Interacts with herpes simplex virus 1 protein ICP34. 5. (Microbial infection) Interacts with Zika virus non-structural protein 1/NS1 and non-structural protein 4B/NS4B. (Microbial infection) Interacts with SARS-CoV-2 non-structural protein 6; this interaction decreases IRF3 phosphorylation by 57%, which leads to reduced IFN-beta (IFNB) production (PubMed:32979938). Interacts with SARS-CoV-2 helicase; this interaction inhibits TBK1 phosphorylation and decreases IRF3 phosphorylation by 75%, which leads to reduced IFN-beta production (PubMed:32979938). Interacts with SARS-CoV-2 M protein; the interaction promotes TBK1 degradation via 'Lys-48'-linked ubiquitination (PubMed:34084167). (Microbial infection) Interacts with human cytomegalovirus protein UL35; this interaction inhibits type I interferon production.
Kinase, Serine/threonine-protein kinase, Transferase
-
- Derivation: Phage display library
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IHC-P, FC, ICC, IF
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: FC, IP, WB
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, IHC, FC
-
- Species Reactivity: Human, Mouse, Rat, Hamster
- Type: Rabbit IgG
- Application: WB
- Mouse Anti-TBK1 Recombinant Antibody (clone 2G1) (VS3-XY1485)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF
-
- Species Reactivity: Human, Hamster, Rat
- Type: Rabbit IgG
- Application: WB
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
For Research Use Only. Not For Clinical Use.

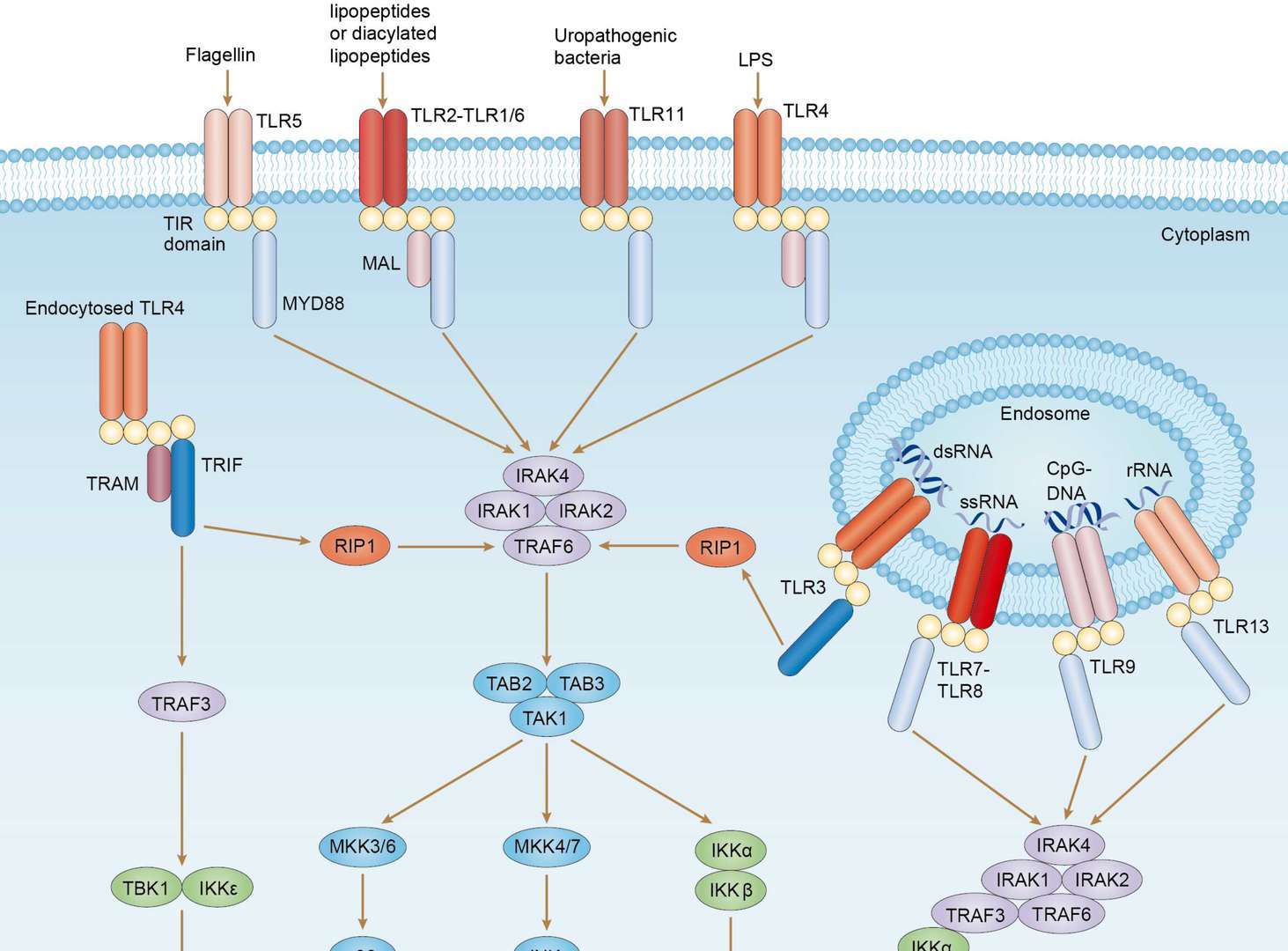 TLR Signaling Pathway
TLR Signaling Pathway