Custom Antibody Production and QC Services
With rich experiences in antibody discovery and development as a full-service CRO, Creative Biolabs possesses extensive expertise in transitioning monoclonal antibodies through the whole product life cycle from concept in early stage development to large-scale Non-GMP commercialization.
Our Experts
To ensure the Non-GMP compliant monoclonal antibody production, our scientists and technicians are trained to fully understand the science and principles behind each process. Our team is dedicated to facilitating your success at every step of the way. We have established a full development package services including process development, Non-GMP antibody manufacturing and quality assurance. These team members are committed to partnering seamlessly to advance your Non-GMP antibody production program.
Our Experiences
Creative Biolabs has been a trusted partner empowered by the highest quality service at competitive price. We have extensive experiences completing Non-GMP antibody production from research quantities to industrial scale in a Non-GMP facility. Creative Biolabs has a long commercial Non-GMP manufacturing history working with several large pharmaceutical companies. Our highly-qualified staff and scientists are confident in providing our clients with a wide range of competences and capabilities.
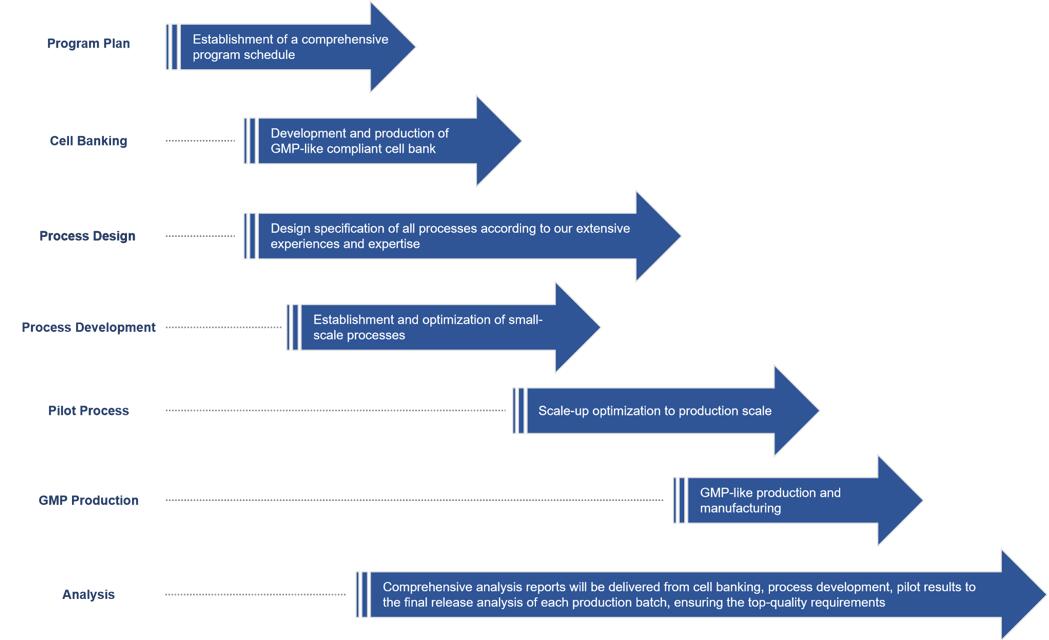
We offer flexible, efficient Non-GMP antibody production services to advance the development of increasing number of therapeutic monoclonal antibody candidates, from process development to aseptic filling. Our process development services include both upstream and downstream processes. To best fit your candidates, we will design specific processes. Our highly flexibility in antibody production and fast implementation of technology changes will ensure the ready-to-use Non-GMP antibody products by integrated sample filling. Here is the whole Non-GMP antibody production services life cycle. Individual module or integrated end-to-end package are all available for our clients.
Our Mission
- We prioritize top-quality Non-GMP antibody manufacturing services by delivering on what we promise.
- Our team members collaboratively work with you to ensure timely data delivery and maximize the success of your project.
- We are committed to ensuring product integrity from beginning to the end.
- Our experts are trained to fully understand the science behind the products.
Creative Biolabs is committed to your antibody drug development and Non-GMP manufacturing requirements. As a full-service CRO, we are dedicated to the development and manufacture of monoclonal antibodies, from early discovery, development, We are making efforts to help you bring novel therapeutic monoclonal antibodies to the market successfully. If you have any questions, please contact us for assistance.
-
OmicsAb™ Prokaryotic Antibody Production Service
- OmicsAb™ Escherichia coli Expression Service offers the most popular system to produce an antibody with low cost and high convenience, especially for antibody fragments.
- OmicsAb™ Bacillus subtilis Expression Service provides an alternative popular system.
-
OmicsAb™ Eukaryotic Antibody Production Service
- OmicsAb™ Yeast Expression Service
- OmicsAb™ Filamentous Fungus Expression Service
- OmicsAb™ Eukaryotic Alga Expression Service
- OmicsAb™ Plant Cell Expression Service
- OmicsAb™ Parasite Expression Service
- OmicsAb™ Insect Cell Expression Service
- OmicsAb™ Mammalian Cell Expression Service
- OmicsAb™ Transgenic Animal Expression Service
- OmicsAb™ High-throughput Antibody Production Service
- Functional Antibody Production and QC Services
- Light-based Antibody Purification Service
- IgA Antibody Engineering & Production Services
- Rare Species Derived Antibody Production & QC Services
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

