PKC Family
The protein kinase C (PKC) family represents a cornerstone in the intricate architecture of cellular signaling, orchestrating a multitude of cellular processes pivotal to the maintenance of homeostasis, proliferation, differentiation, and apoptosis. Comprising a diverse group of phospholipid-dependent serine/threonine kinases, the twelve mammalian PKC family members are usually classified into four categories based on their activation requirements: classical PKCs (alpha, betaI, betaII, gamma), novel PKCs (delta, epsilon, eta, theta), atypical PKCs (zeta, iota/lambda), and the PKNs (PKN1, PKN2 and PKN3). Each category, through its unique activation mechanisms, tailors the cellular response to various extracellular signals, thereby underscoring the versatility and specificity of PKC-mediated pathways. The role of PKCs extends beyond mere signal transduction; they are central to the modulation of cellular architecture, the dynamics of vesicle trafficking, and the fine-tuning of gene expression. Crucially, aberrations in PKC signaling have been implicated in a wide array of diseases, ranging from cancer to neurological disorders and cardiovascular diseases. In cancer, PKC isoforms can act as oncogenes or tumor suppressors, depending on their context and mode of activation. In neurological disorders, altered PKC activity has been linked to disrupted neuronal signaling and plasticity. Similarly, in cardiovascular diseases, PKCs play critical roles in the regulation of cardiac hypertrophy and contractility. Thus, the PKC family emerges not only as a fundamental component of cellular signaling but also as a potential therapeutic target, where modulation of specific PKC isoforms could pave the way for innovative treatments across a spectrum of diseases.
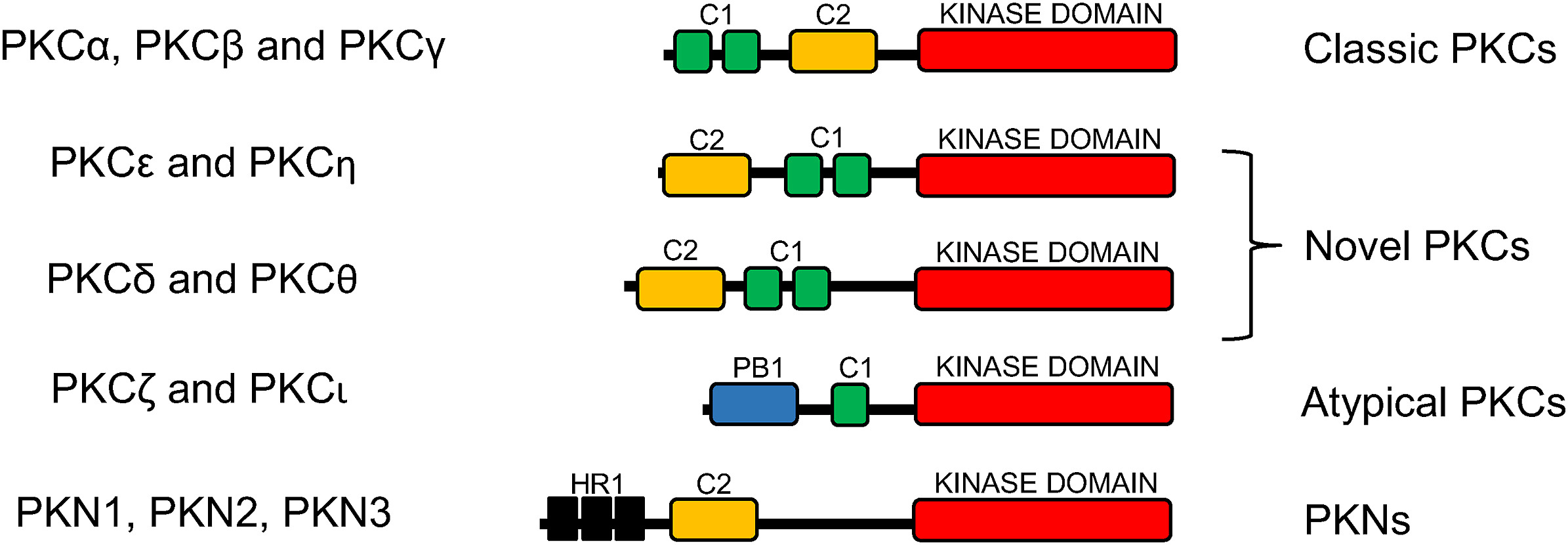 Figure 1 Structure and classification of PKC subfamilies. (Garcia, 2021)
Figure 1 Structure and classification of PKC subfamilies. (Garcia, 2021)
Representative Targets in PKC Family
PRKCA
Protein Kinase C alpha (PRKCA) is a pivotal enzyme in the classical Protein Kinase C (PKC) family, which plays a critical role in the transduction of signals received at the cell surface into cellular responses. PRKCA is activated by the binding of diacylglycerol (DAG) and calcium ions, subsequently translocating from the cytosol to the plasma membrane where it becomes active. Once activated, PRKCA influences several pathways that govern cellular life and death decisions, demonstrating its importance in maintaining cellular homeostasis. Its roles extend to the regulation of gene expression, modulation of membrane receptor function, and the activation of other enzymes that are crucial in metabolic pathways. Importantly, PRKCA has been implicated in the pathogenesis of various diseases, including cancer, cardiovascular diseases, and neurological disorders, highlighting its significance not only in normal cellular physiology but also in disease states.


PRKCB
Protein kinase C beta (PRKCB) is another serine/threonine kinase that belongs to the classical PKC family. Same as PRKCA, PRKCB is activated through various mechanisms, including the interaction with phospholipids, calcium ions, and diacylglycerol (DAG), thereby functioning as a critical mediator in signaling pathways. PRKCB exists in two isoforms, PRKCB1 and PRKCB2, which are generated through alternative splicing and exhibit distinct tissue distributions, thereby enabling fine-tuned responses to cellular signals. Its roles are multifaceted, encompassing the regulation of gene expression, modulation of the cell cycle, and influencing the pathophysiology of several diseases such as diabetes, cardiovascular diseases, and cancer.


PRKCZ
Protein kinase C zeta type (PRKCZ) is an integral member of the PKC family of serine/threonine kinases, distinguished by its unique structure and activation mechanisms. Unlike classical PKCs that require calcium and diacylglycerol for activation, PRKCZ is part of the atypical PKCs, which are independent of these activators and are stimulated by protein-protein interactions and phospholipids. This kinase plays pivotal roles in diverse cellular processes, including regulation of cell survival and apoptosis, control of gene expression, and modulation of cell adhesion and migration. It is particularly notable for its involvement in the PI3K/Akt signaling pathway, where it functions downstream of PI3K and contributes to the regulation of metabolism, cell growth, and survival. Moreover, PRKCZ has been implicated in the establishment and maintenance of cell polarity, essential for processes such as neuron differentiation and epithelial tissue organization.


Full List of Targets in PKC Family
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| PRKCA | PRKCA; PKCA; PRKACA; Protein kinase C alpha type; PKC-A; PKC-alpha | 5578 | P17252 | PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. The protein encoded by this gene is one of the PKC family members. This kinase has been reported to play roles in many different cellular processes, such as cell adhesion, cell transformation, cell cycle checkpoint, and cell volume control. Knockout studies in mice suggest that this kinase may be a fundamental regulator of cardiac contractility and Ca(2+) handling in myocytes. |
| PRKCB | PRKCB; PKCB; PRKCB1; Protein kinase C beta type; PKC-B; PKC-beta | 5579 | P05771 | Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by calcium and second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play a distinct role in cells. The protein encoded by this gene is one of the PKC family members. This protein kinase has been reported to be involved in many different cellular functions, such as B cell activation, apoptosis induction, endothelial cell proliferation, and intestinal sugar absorption. Studies in mice also suggest that this kinase may also regulate neuronal functions and correlate fear-induced conflict behavior after stress. Alternatively spliced transcript variants encoding distinct isoforms have been reported. |
| PRKCD | Pkcd; PKC[d]; AI385711; PKCdelta; D14Ertd420e | 5580 | Q05655 | Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by the second messenger diacylglycerol. |
| PRKCE | PKCE; nPKC-epsilon; PRKCE | 5581 | L7RTI5 | Protein kinase C (PKC) is a family of serine- and threonine-specific protein kinases that can be activated by calcium and the second messenger diacylglycerol. PKC family members phosphorylate a wide variety of protein targets and are known to be involved in diverse cellular signaling pathways. PKC family members also serve as major receptors for phorbol esters, a class of tumor promoters. Each member of the PKC family has a specific expression profile and is believed to play a distinct role in cells. The protein encoded by this gene is one of the PKC family members. This kinase has been shown to be involved in many different cellular functions, such as neuron channel activation, apoptosis, cardioprotection from ischemia, heat shock response, as well as insulin exocytosis. Knockout studies in mice suggest that this kinase is important for lipopolysaccharide (LPS)-mediated signaling in activated macrophages and may also play a role in controlling anxiety-like behavior. |
| PRKCI | Protein Kinase C Iota; Protein Kinase C, Iota; Atypical Protein Kinase C-Lambda/Iota; PRKC-Lambda/Iota; APKC-Lambda/Iota; EC 2.7.11.13; NPKC-Iota; DXS1179E; EC 2.7.11; PKCI | 5584 | P41743 | Protein kinase C iota type is an enzyme in humans encoded by the PRKCI gene,which encodes a member of the protein kinase C (PKC) family of serine/threonine protein kinases. The PKC family comprises at least eight members, which are differentially expressed and are involved in a wide variety of cellular processes. This protein kinase is calcium-independent and phospholipid-dependent that can not get activated by phorbolesters or diacylglycerol. This kinase can be recruited to vesicle tubular clusters (VTCs) by direct interaction with the small GTPase RAB2, where this kinase phosphorylates glyceraldehyde-3-phosphate dehydrogenase (GAPD/GAPDH) plays a role in microtubule dynamics in the early secretory pathway. PRKCI is found to be necessary for BCL-ABL-mediated resistance to drug-induced apoptosis and therefore protects leukemia cells against drug-induced apoptosis. Associated diseases include Glioblastoma and Lung Squamous Cell Carcinoma. Its related pathways are NFAT and Cardiac Hypertrophy and GPCR Pathway. |
| PRKCZ | PKC2; PKC-ZETA | 5590 | Q05513 | Protein kinase C (PKC) zeta is a member of the PKC family of serine/threonine kinases which are involved in a variety of cellular processes such as proliferation, differentiation and secretion. Unlike the classical PKC isoenzymes which are calcium-dependent, PKC zeta exhibits a kinase activity which is independent of calcium and diacylglycerol but not of phosphatidylserine. Furthermore, it is insensitive to typical PKC inhibitors and cannot be activated by phorbol ester. Unlike the classical PKC isoenzymes, it has only a single zinc finger module. These structural and biochemical properties indicate that the zeta subspecies is related to, but distinct from other isoenzymes of PKC. Alternative splicing results in multiple transcript variants encoding different isoforms. |
Tested Data-Supported Products for Targeting PKC Family
- Garcia-Concejo, Adrian, and Dan Larhammar. "Protein kinase C family evolution in jawed vertebrates." Developmental Biology 479 (2021): 77-90. Distributed under Open Access license CC BY 4.0, without modification.
 Loading...
Loading...- AbPlus™ Anti-PRKCB Magnetic Beads (CBACN-451) (VS-0424-XY217)
-
- Target: PRKCB
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-PRKCA Magnetic Beads (CBACN-450) (VS-0424-XY216)
-
- Target: PRKCA
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC, ICC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IP
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IHC, IP, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IHC, IP, FC
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: WB, ELISA
-
- Derivation: Rabbit
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ELISA
-
- Derivation: Rabbit
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ELISA
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, WB, IF
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: FC
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: FC
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: FC
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: ELISA, WB, IP, ICC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: ELISA, WB, IP, ICC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, IP
- Rabbit Anti-PRKCB Polyclonal Antibody (MRO-2124-CN) (MRO-2124-CN)
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IF, IHC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, IP, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, IP, FC
-
- Species Reactivity: Human
- Application: ICC, IF, IHC-P, WB
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: IHC-Fr
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1, κ
- Application: WB, ELISA, IF, IP
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG2a
- Application: WB, ELISA
- Rabbit Anti-NHP PRKCE Recombinant Antibody (VS-1024-XY391) (VS-1024-XY391)
-
- Species Reactivity: Non-human primate, Rat, Human, Mouse
- Type: Rabbit IgG
- Application: WB
- Mouse Anti-NHP PRKCA Recombinant Antibody (clone MC5) (VS-1024-XY390)
-
- Species Reactivity: Bovine, Dog, Human, Mouse, Non-human primate, Rabbit, Rat
- Type: Mouse IgG2a
- Application: WB, IHC, IF, IP
-
- Scaffold Name: Receptor for activated C-kinase 1 (RACK1)
- Target: Protein kinase C (PKC)
- Species Reactivity: Human
- Derivation: Phage display
-
- Species Reactivity: Human
- Type: Llama VHH
- Application: WB, IHC, FC, FuncS
- Anti-Human PRKCB Immunohistochemistry Kit (VS-0525-XY5690)
-
- Species Reactivity: Human
- Target: PRKCB
- Application: IHC
- Anti-Rat PRKCA Immunohistochemistry Kit (VS-0525-XY5689)
-
- Species Reactivity: Human, Mouse, Rat
- Target: PRKCA
- Application: IHC
- Anti-Sheep PRKCA Immunohistochemistry Kit (VS-0525-XY5688)
-
- Species Reactivity: Human, Mouse, Rat, Monkey, Sheep, Zebrafish
- Target: PRKCA
- Application: IHC
- Anti-Human PRKCA Immunohistochemistry Kit (VS-0525-XY5686)
-
- Species Reactivity: Human
- Target: PRKCA
- Application: IHC
- Anti-Mouse PRKCE Immunohistochemistry Kit (VS-0525-XY5695)
-
- Species Reactivity: Human, Mouse, Rat, Monkey
- Target: PRKCE
- Application: IHC
- Anti-PRKCE Immunohistochemistry Kit (VS-0525-XY5694)
-
- Species Reactivity: Human
- Target: PRKCE
- Application: IHC
- Anti-Mouse PRKCB Immunohistochemistry Kit (VS-0525-XY5691)
-
- Species Reactivity: Human, Mouse, Rat
- Target: PRKCB
- Application: IHC
- Anti-Mouse PRKCA Immunohistochemistry Kit (VS-0525-XY5687)
-
- Species Reactivity: Human, Mouse, Rat, Fruit fly
- Target: PRKCA
- Application: IHC
- Anti-PRKCB Immunohistochemistry Kit (VS-0325-XY1750)
-
- Species Reactivity: Human, Mouse, Rat
- Target: PRKCB
- Application: IHC
- Anti-PRKCA Immunohistochemistry Kit (VS-0325-XY1749)
-
- Species Reactivity: Human, Mouse, Rat
- Target: PRKCA
- Application: IHC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IHC-P, FC
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, ICC, IF, IHC-P, IP, FC
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-P, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IHC-P, FC, IP
-
- Derivation: Phage display library screening
- Type: IgG
- Application: WB, IP, ELISA, IHC-FoFr
-
- Derivation: Phage display library screening
- Species Reactivity: Human
- Type: IgG
- Application: IHC-P, WB, ICC
-
- Derivation: Phage display library screening
- Species Reactivity: Human
- Type: IgG
- Application: WB, IP, IHC-P, ICC, IF
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
For Research Use Only. Not For Clinical Use.

















