Signal Transduction in Cancer
Signal transduction in cancer encompasses a complex network of cellular processes and pathways that are pivotal in the initiation, progression, and metastasis of cancer. At its core, signal transduction involves the conversion of external signals into cellular responses, a fundamental aspect of cellular biology that governs cell growth, differentiation, and survival. In the context of cancer, these normally tightly regulated processes become dysregulated, leading to aberrant cellular behaviors that underpin tumor development and proliferation. This dysregulation can occur through various mechanisms, including mutations in genes encoding for signal transduction proteins, overexpression of receptor proteins on the cell surface, and alterations in downstream signaling molecules and pathways. These changes result in the continuous activation of signaling pathways that drive malignant transformation, sustain tumor growth, and enable cancer cells to evade apoptosis, invade surrounding tissues, and ultimately metastasize to distant organs. By interrupting these signaling pathways, researchers aim to halt the proliferation of cancer cells and induce apoptosis, thereby offering hope for more effective and less toxic cancer treatments.
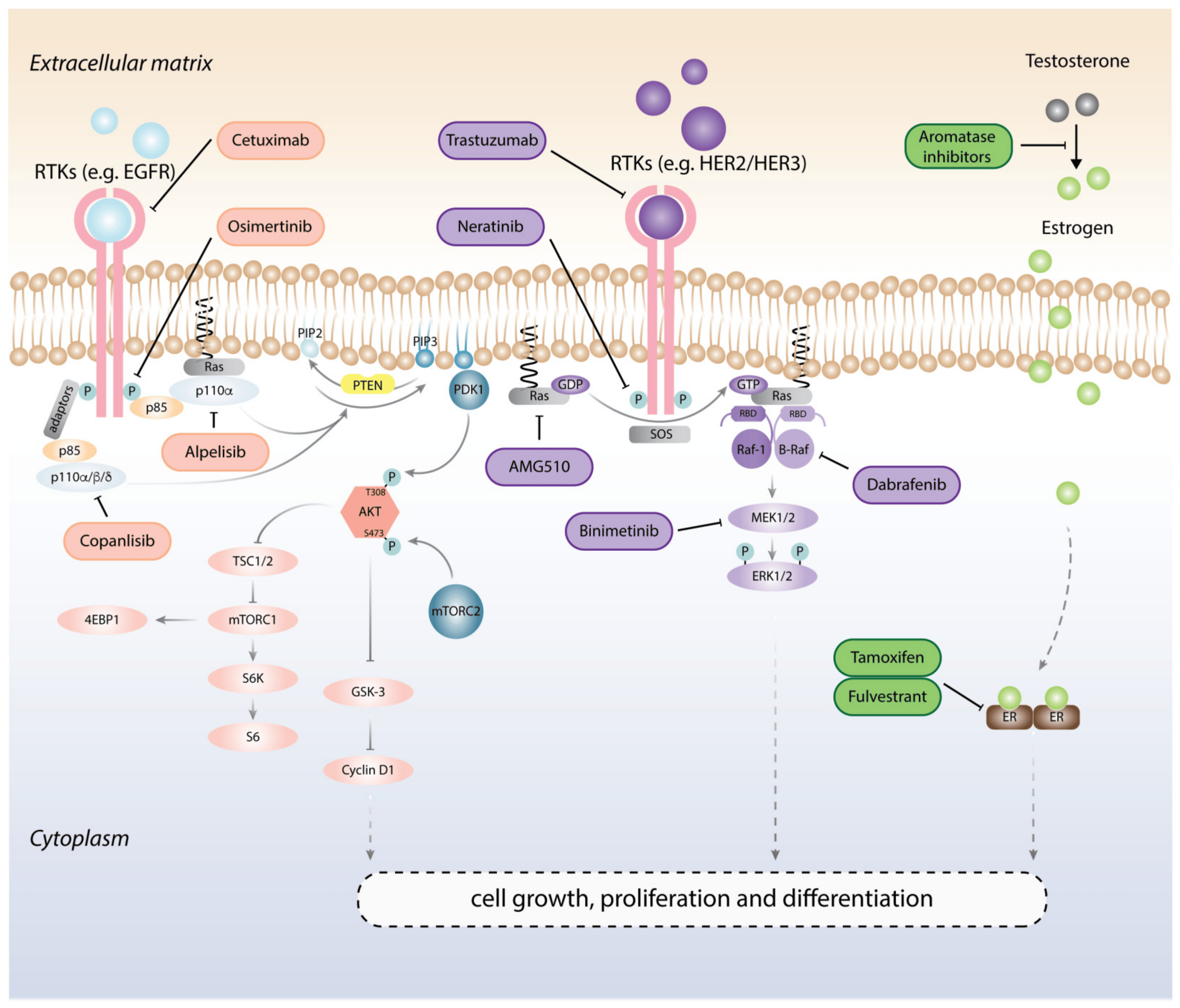 Figure 1 Targeted therapies for cancer treatment. (Yip, 2021)
Figure 1 Targeted therapies for cancer treatment. (Yip, 2021)
FAK-Src Signaling
Focal Adhesion Kinase (FAK) and Src are pivotal components of cell signaling pathways that play a critical role in the regulation of cellular processes such as proliferation, survival, migration, and invasion, which are instrumental in cancer progression and metastasis. The FAK-Src signaling axis is particularly notable for its involvement in the modulation of the tumor microenvironment, influencing tumor growth and the spread of cancer cells to distant organs. This signaling pathway is activated by integrins and growth factor receptors, leading to the phosphorylation and activation of FAK and Src. The consequent activation of downstream signaling cascades can result in the alteration of gene expression and the modulation of cellular functions that are crucial for cancer progression.
PI3K Pathway
The PI3K (phosphoinositide 3-kinase) pathway is a critical signaling cascade that plays a fundamental role in various cellular processes, including growth, metabolism, survival, and proliferation. In cancer, the deregulation of this pathway is a pivotal event that contributes to the initiation, progression, and maintenance of tumor growth, and has emerged as a key target for the development of novel anticancer therapeutics, with a significant effort being directed towards identifying and characterizing inhibitors that can effectively disrupt this pathway in cancer cells.
PKC Family
The Protein Kinase C (PKC) family comprises a group of serine/threonine kinases that play pivotal roles in cell signaling pathways. These enzymes are integral to the regulation of diverse cellular processes, including proliferation, differentiation, and apoptosis. PKC enzymes have garnered significant attention due to their dualistic roles as promoters and suppressors of tumorigenesis, depending on the cellular context and specific PKC isoform involved. Their involvement in cancer is multifaceted, influencing not only cell growth and survival but also angiogenesis, metastasis, and drug resistance.
RAS-MAPK Pathway
The RAS-MAPK pathway plays a pivotal role in the regulation of cell growth, differentiation, and survival. Central to this pathway are the RAS GTPases, which act as molecular switches, and the downstream mitogen-activated protein kinases (MAPKs) that propagate signals to influence cellular responses. In cancer, aberrations in the RAS-MAPK pathway are frequently observed, making it a focal point of oncogenic research and therapeutic target development. Mutations in the RAS genes or other components of the pathway can lead to uncontrolled cell proliferation and survival, hallmark features of cancerous cells. Given its significance, the pathway has been extensively studied, revealing its complexity and the multitude of mechanisms through which it can influence oncogenesis.
Rho GTPases
The Rho GTPases pathway, a critical subset of the Ras superfamily, plays a pivotal role in regulating various cellular processes, including cytoskeletal organization, cell proliferation, apoptosis, migration, and adhesion. These functions are crucial for maintaining normal cellular activities but, when dysregulated, can contribute significantly to cancer progression and metastasis. Alterations in the Rho GTPases pathway can lead to aberrant cell growth, evasion of programmed cell death, enhanced migratory capacity, and ultimately, tumor metastasis. The complex interplay between Rho GTPases and their regulators thus represents a promising therapeutic target, offering potential strategies for cancer treatment through the modulation of this pathway.
Ubiquitin-Proteasome Pathway
The ubiquitin-proteasome pathway represents a critical mechanism in cellular regulation, instrumental in maintaining homeostasis through the targeted degradation of proteins. This complex biochemical pathway is pivotal in controlling a wide array of cellular processes, including cell cycle progression, transcriptional regulation, DNA repair, and apoptosis. Aberrations in the ubiquitin-proteasome system can lead to the unchecked growth of cells, evasion of apoptosis, and enhanced metastatic potential, contributing to tumor development and progression. By targeting specific components of the ubiquitin-proteasome pathway, researchers aim to develop novel anticancer strategies that could potentially inhibit tumor growth, induce cancer cell death, and overcome drug resistance, marking the UPP as a promising target in the ongoing battle against cancer.
- Yip, Hon Yan Kelvin, and Antonella Papa. "Signaling pathways in cancer: therapeutic targets, combinatorial treatments, and new developments." Cells 10.3 (2021): 659. Distributed under Open Access license CC BY 4.0, without modification.
 Loading...
Loading...- AbPlus™ Anti-PSMA1 Magnetic Beads (VS-0724-YC1398) (VS-0724-YC1398)
-
- Target: PSMA1
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-HRAS Magnetic Beads (VS-0724-YC1294) (VS-0724-YC1294)
-
- Target: HRAS
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-Uchl1 Magnetic Beads (VS-0724-YC1237) (VS-0724-YC1237)
-
- Target: Uchl1
- Target Species: Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-CDC34 Magnetic Beads (VS-0724-YC1215) (VS-0724-YC1215)
-
- Target: CDC34
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-MAP2K2 Magnetic Beads (VS-0724-YC1183) (VS-0724-YC1183)
-
- Target: MAP2K2
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-UCHL1 Magnetic Beads (VS-0724-YC1164) (VS-0724-YC1164)
-
- Target: UCHL1
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-Uchl1 Magnetic Beads (VS-0724-YC1162) (VS-0724-YC1162)
-
- Target: Uchl1
- Target Species: Mouse
- Application: IP, Protein Purification
- AbPlus™ Anti-PIK3R1 Magnetic Beads (VS-0724-YC1064) (VS-0724-YC1064)
-
- Target: PIK3R1
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-RHOA Magnetic Beads (VS-0724-YC730) (VS-0724-YC730)
-
- Target: RHOA
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-CDC42 Magnetic Beads (VS-0724-YC659) (VS-0724-YC659)
-
- Target: CDC42
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-MLST8 Magnetic Beads (VS-0724-YC593) (VS-0724-YC593)
-
- Target: MLST8
- Target Species: Human
- Application: IP, Protein Purification
- AbPlus™ Anti-USP7 Magnetic Beads (D545) (VS-0424-XY271)
-
- Target: USP7
- Target Species: Human
- Application: IP-MS, Protein Purification
- AbPlus™ Anti-UCHL1 Magnetic Beads (JF93-08) (VS-0424-XY270)
-
- Target: UCHL1
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-RAC1 Magnetic Beads (JF10-11) (VS-0424-XY221)
-
- Target: RAC1
- Target Species: Human, Mouse, Rat, Zebrafish
- Application: IP, Protein Purification
- AbPlus™ Anti-PTK2 Magnetic Beads (CBACN-222) (VS-0424-XY219)
-
- Target: PTK2
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-PSMA1 Magnetic Beads (JB89-30) (VS-0424-XY218)
-
- Target: PSMA1
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-PRKCB Magnetic Beads (CBACN-451) (VS-0424-XY217)
-
- Target: PRKCB
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-PRKCA Magnetic Beads (CBACN-450) (VS-0424-XY216)
-
- Target: PRKCA
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-NRAS Magnetic Beads (JF10-11) (VS-0424-XY205)
-
- Target: NRAS
- Target Species: Human, Mouse, Rat, Zebrafish
- Application: IP, Protein Purification
- AbPlus™ Anti-MAPK3 Magnetic Beads (CBACN-216) (VS-0424-XY181)
-
- Target: MAPK3
- Target Species: Human, Mouse
- Application: IP, Protein Purification
- AbPlus™ Anti-AKT1 Magnetic Beads (CBACN-016) (VS-0424-XY10)
-
- Target: AKT1
- Target Species: Human, Mouse, Rat
- Application: IP, Protein Purification
- Mouse Anti-UCHL1 Recombinant Antibody (VS3-FY2912) (VS3-FY2912)
-
- Species Reactivity: Human, Monkey
- Type: Mouse IgG
- Application: WB, ICC
- Mouse Anti-RAC1 Recombinant Antibody (VS3-FY2857) (VS3-FY2857)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Mouse IgG
- Application: WB, IHC, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC, ICC
- Rabbit Anti-PIK3R1 Recombinant Antibody (VS3-FY2835) (VS3-FY2835)
-
- Species Reactivity: Human, Rat, Hamster
- Type: Rabbit IgG
- Application: WB, ICC, IP
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IHC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ELISA
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, ELISA
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF
-
- Type: Rabbit IgG
- Application: WB, ICC, IF
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, IP
-
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IP
- Rabbit Anti-AKT1 Recombinant Antibody (VS3-FY2622) (VS3-FY2622)
-
- Species Reactivity: Human, Mouse, Rat, Hamster
- Type: Rabbit IgG
- Application: WB, IHC, ICC, IP
-
- Species Reactivity: Mouse
- Type: Rabbit IgG
- Application: ELISA
-
- Species Reactivity: Mouse
- Type: Rabbit IgG
- Application: ELISA
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA
- Mouse Anti-RHOA Recombinant Antibody (clone 4E12) (VS3-WK1600)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Mouse IgG
- Application: IHC-P, WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, IP, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, IP, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-P, IP, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IF, FC
-
- Species Reactivity: Human, Mouse, Rat, Hamster
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-Fr, IHC-P, IF, IP
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IP
- Human Anti-AKT1 Recombinant Antibody (clone 30769) (VS-0224-XY5)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: WB
- Recombinant Mouse Anti-UCHL1 Antibody (VS-0923-FY154) (VS-0923-FY154)
-
- Species Reactivity: Human
- Type: Mouse IgG2a
- Application: ELISA, WB
- Recombinant Mouse Anti-MTOR Antibody (VS-0923-FY112) (VS-0923-FY112)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB
- Recombinant Mouse Anti-CDC42 Antibody (VS-0923-FY37) (VS-0923-FY37)
-
- Species Reactivity: Human
- Type: Mouse IgG2b
- Application: ELISA, WB
- Recombinant Mouse Anti-CDC42 Antibody (VS-0923-FY36) (VS-0923-FY36)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB
- Recombinant Mouse Anti-AKT1 Antibody (VS-0923-FY6) (VS-0923-FY6)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB
- Rabbit Anti-UCHL1 Recombinant Antibody (VS-0723-WK91) (VS-0723-WK91)
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA
-
- Species Reactivity: Human
- Type: Mouse IgG3
- Application: IHC-P
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-Fr, IHC-P, ICC, IF, IP
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IHC-P
- Recombinant Mouse Anti-UCHL1 Antibody (clone 1D1B12) (VS3-FY1518)
-
- Type: Mouse IgG2b
- Application: ELISA, WB, IHC, FC
- Recombinant Mouse Anti-UCHL1 Antibody (clone 1B4H3) (VS3-FY1517)
-
- Type: Mouse IgG2b
- Application: ELISA, WB, IHC, FC
- Recombinant Rabbit Anti-SRC Antibody (clone R05-7H8) (VS3-FY1389)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IP
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ICC, IF, IP
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Type: Mouse IgG1
- Application: ELISA, FC
-
- Type: Mouse IgG2b
- Application: ELISA, IHC, FC
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-Fr, IHC-P, ICC, IF, IP
-
- Derivation: Mouse
- Species Reactivity: Human, Mouse, Pig, Rat
- Type: Mouse IgG2b
- Application: ELISA, WB
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, WB, IF
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
For Research Use Only. Not For Clinical Use.

