Wheat Germ Cell-Free based Antibody Production and QC Service
Eukaryotic ribosomes from plants are more suited for eukaryotic protein production than prokaryotic ribosomes from E. coli extracts. High-performance wheat germ extracts have demonstrated the highest protein expression activity among eukaryotic systems, which has led to the system's widespread application in applied sciences and research. The germ provides a remarkably abundant source of the protein components and ribosomes required for quick protein synthesis from stored mRNAs during early germination. This system is suitable for the production of proteins with complicated conformation and multiple protein complexes, such as disulfide-bond-containing or integral membrane proteins.
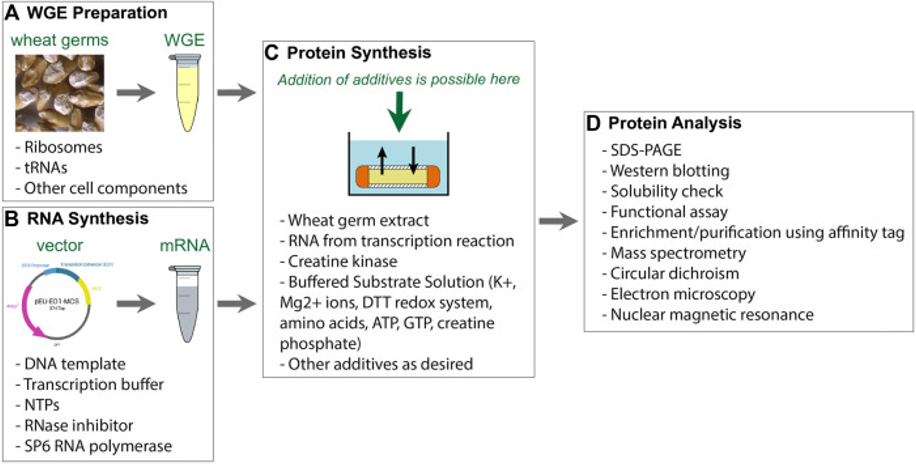 Fig.1. Workflow of wheat germ cell-free system.1
Fig.1. Workflow of wheat germ cell-free system.1
Antibody Production in Wheat Germ Cell-Free Systems
One issue that frequently arises in cell-free systems is the need for disulfide bonds in proteins, such as antibodies. Disulfide bonds cannot develop because the original extract contains the redox agent dithiothreitol (DTT) in some cell-free systems. In wheat-germ extracts, this restriction was removed by taking advantage of the open structure of cell-free systems. The synthesis of a functional single-chain antibody variable fragment (scFv) aided in the creation of disulfide bonds by lowering the DTT concentration and adding protein disulfide isomerase. Subsequently, this strategy was expanded by using both reduced and oxidized glutathione, which further improved the conditions for disulfide bond formation. Additionally, a wheat-germ system was used to produce malaria proteins to find new vaccine candidates.
Advantages of Wheat Germ Cell-Free Systems
- High yield of complex proteins
- Systems accessible for disulfide-bridged protein synthesis
- Proper folding of proteins and high protein solubility
Applications of Wheat Germ Cell-Free Systems
Chaperone molecules like GroEL and GroES, whose inclusion seems necessary to enable proper disulfide bond formation in E. coli cell-free systems, do not need to be added to the wheat germ cell-free system. A scFv antibody fragment binding to Salmonella O-antigen was used as a model protein. For the first time, this scFv fragment was successfully made using an E. coli expression system, where the polypeptide that was synthesized was released into the bacteria's periplasm. However, the scFv produced in the wheat-germ cell-free system has a higher affinity than that produced in the E. coli system, according to the results of the antigen-binding analysis. The eukaryotic and prokaryotic translation systems differ in certain inherent features, which could account for the disparity in the produced polypeptide's activity.
Creative Biolabs provides recombinant antibody production services in wheat germ cell-free systems. With our vast experience and cutting-edge technological platforms, our one-stop integrated services can support our global clients' varied research and projects. Please feel free to contact us for more details.
Reference
- Fogeron, Marie-Laure et al. “Easy Synthesis of Complex Biomolecular Assemblies: Wheat Germ Cell-Free Protein Expression in Structural Biology.” Frontiers in molecular biosciences vol. 8 639587. 25 Mar. 2021, doi:10.3389/fmolb.2021.639587. Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

