ScFv-Fc Fusion Antibody Production and QC Service
Recombinant antibodies are increasingly utilized in targeted therapy and immunotherapy. As a leading antibody supplier, Creative Biolabs has established a high-throughput platform for the expression and purification of ScFv-Fc recombinant antibodies. Our platform aids clients in accelerating their antibody drug development processes across various stages, reducing costs, and enhancing success rates. We offer a variety of expression systems, including options such as E. coli, yeast, insect cells, and mammalian cells. Simultaneously, we use cutting-edge bioreactor technology to ensure optimal yield and purity.
Introduction
ScFv-Fc antibodies combine the advantageous properties with the functional benefits of the Fc region. The ScFv fragment, characterized by its smaller molecular weight, enables rapid tissue penetration and effective targeting of antigens, showcasing high adaptability. Meanwhile, the Fc portion contributes to a prolonged half-life of the antibody and enhances immune responses, such as the activation of antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC). Creative Biolabs is capable of developing and producing recombinant ScFv-Fc antibodies, allowing for more efficient targeting and elimination of tumor cells or other pathogens. All of our ScFv-Fc recombinant antibody products exhibit high specificity and affinity, minimizing damage to normal cells while improving therapeutic efficacy.
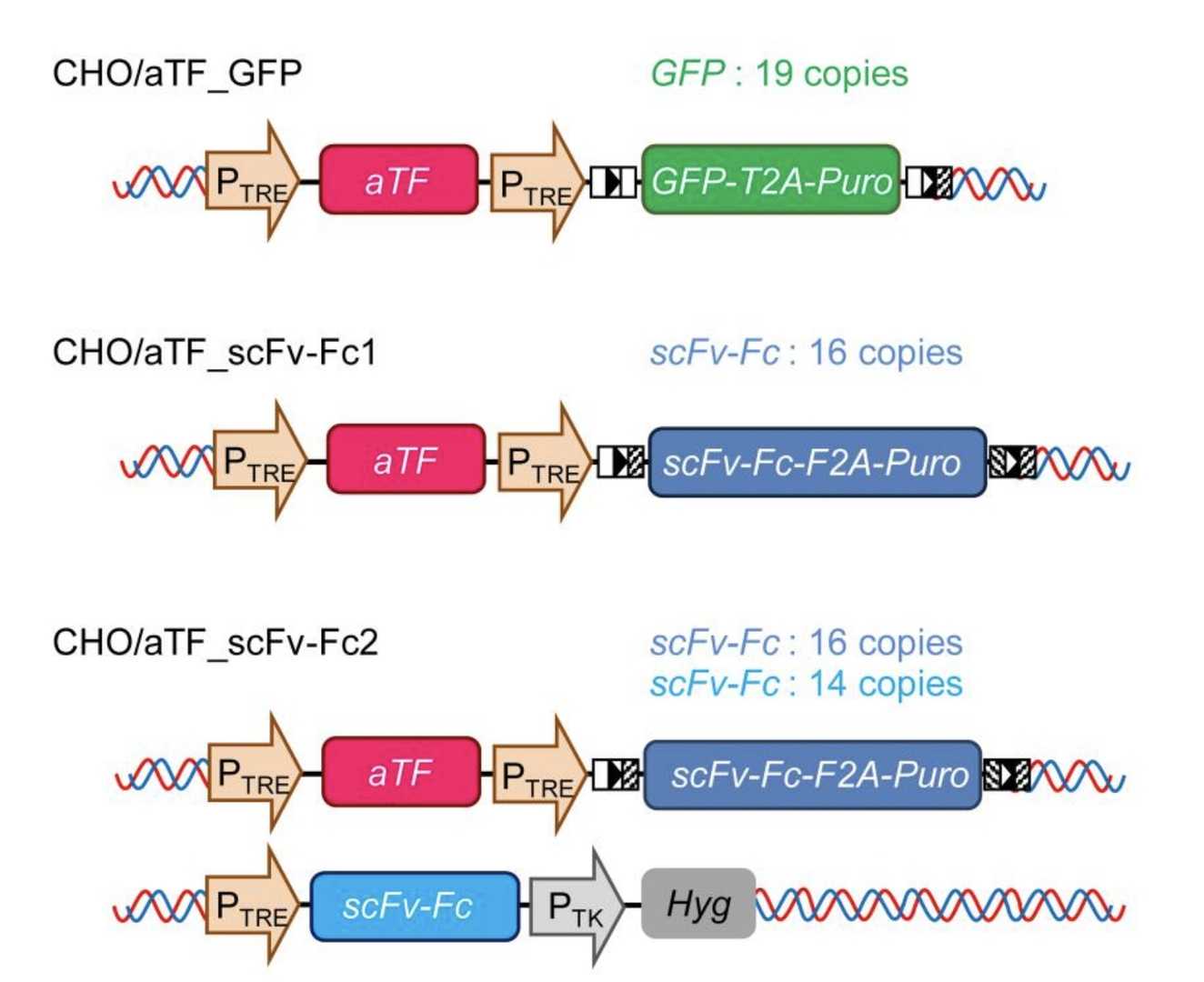 Fig.1 The scFv-Fc Production.1,3
Fig.1 The scFv-Fc Production.1,3
Services
At our company, Creative Biolabs offers a wide array of efficient services for the expression and purification of ScFv-Fc recombinant antibodies, focusing on the following areas:
- Gene Cloning and Construction: Utilizing advanced molecular cloning techniques, we swiftly construct ScFv-Fc genes to meet your specific requirements.
- High-Efficiency Expression Systems: We employ a range of expression systems, including E. coli, yeast, and mammalian cells, to customize high-yield and high-quality ScFv-Fc antibodies for your needs.
- Purification Optimization: With extensive purification experience, we select the most suitable purification methods—such as affinity chromatography and ion exchange chromatography—based on the characteristics of your target molecule to ensure the activity and purity of the antibodies.
- Functional Assessment: We provide evaluations of antibody affinity, specificity, and biological activity, guaranteeing that your ScFv-Fc antibodies are suitable for subsequent research applications.
- Technical Support and Consultation Services: Our team of experts is dedicated to providing you with comprehensive technical support to ensure the smooth progress of your project.
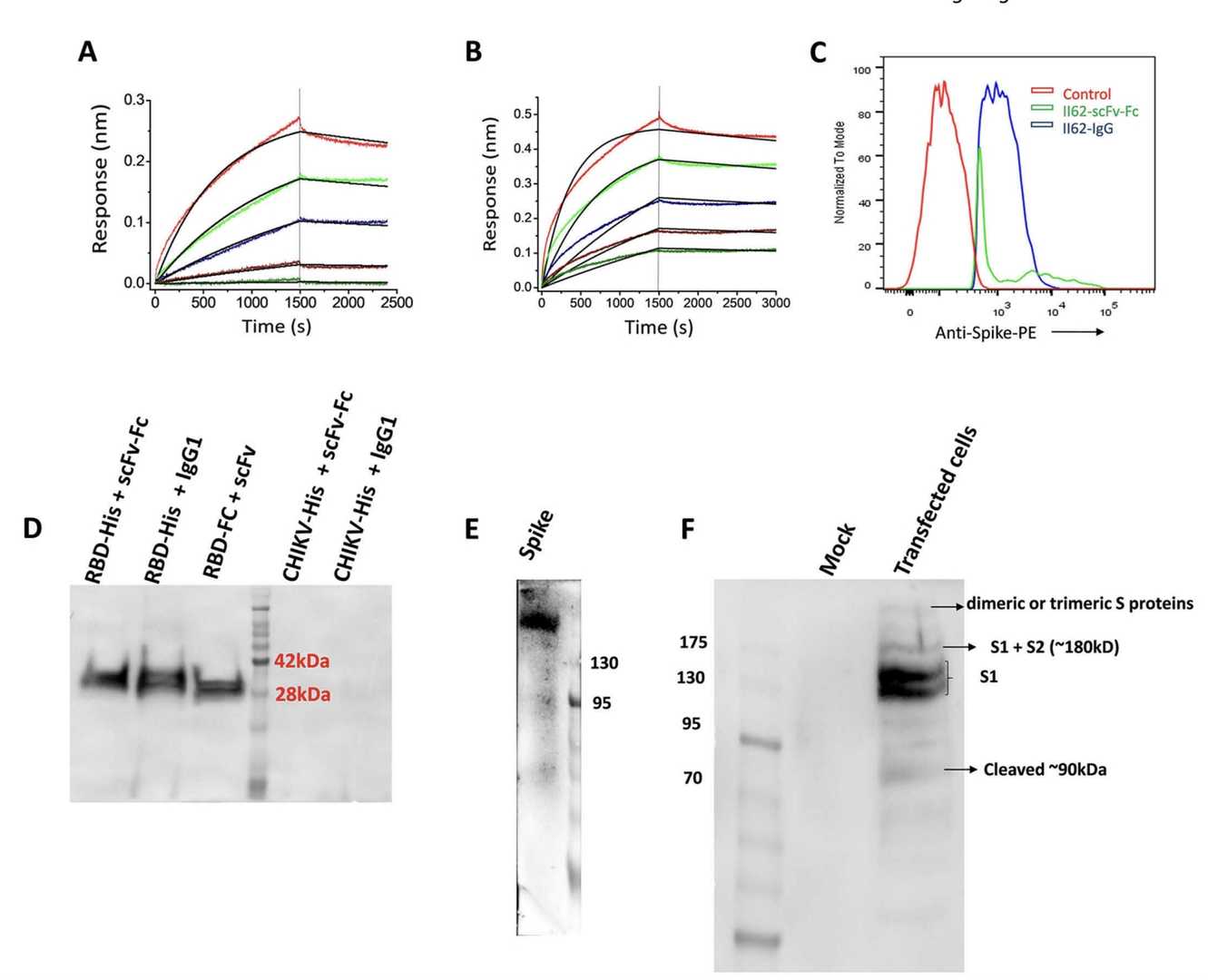 Fig.2 The Binding Analysis of ScFv-Fc Antibodies.2,3
Fig.2 The Binding Analysis of ScFv-Fc Antibodies.2,3
Case Study
a. Primary Objectives of the Project
1. To optimize the expression system of ScFv-Fc antibodies, enhancing both yield and purity.
2. To develop an efficient purification process that ensures the antibodies meet the quality standards for downstream applications.
3. To provide comprehensive technical support and data analysis, offering a solid foundation for subsequent development by clients.
b. Project Workflow
Upon project initiation, our technical team promptly embarked on a series of research and experiments. Initially, we evaluated different expression hosts (such as E. coli, yeast, and mammalian cells) to identify the most suitable expression system. After several rounds of experimentation, we selected mammalian cells as our expression platform to guarantee proper folding and glycosylation of the antibodies.
Next, we systematically optimized the expression conditions, including factors like induction temperature, concentration of inducers, and culture duration, aiming to maximize the yield of ScFv-Fc antibodies. Additionally, we employed molecular biology techniques to construct an improved expression vector, further enhancing the solubility of the antibodies.
Following antibody expression, we designed an efficient purification workflow that included affinity chromatography, ion exchange chromatography, and ultrafiltration concentration, to achieve high-purity recombinant antibodies. Each parameter in these steps was meticulously optimized to ensure the final product met quality standards.
c. Project Outcome
After several weeks of close collaboration, we successfully delivered ScFv-Fc antibody samples with excellent purity (>95%) and high biological activity. In subsequent cell experiments, the client found that the optimized antibody demonstrated superior binding affinity and remarkable anti-tumor activity, far exceeding initial expectations.
- Increased Expression Levels: The optimized expression system has resulted in a 30% increase in the yield of ScFv-Fc antibodies compared to traditional methods, providing customers with a greater output.
- High Purity: Our refined purification process has achieved a product purity of 98.83%, far exceeding industry standards and ensuring the effectiveness and safety of the antibodies for subsequent applications.
- Efficiency Improvement: The overall production cycle has been reduced from several weeks to just 10 days, significantly enhancing research and development efficiency for our clients and accelerating their progress into clinical trial phases.
Advantages
In recent years, recombinant antibodies have become increasingly prevalent in the field of biomedicine, serving as powerful tools for treating a variety of diseases. With ongoing advancements in technology, ScFv-Fc has emerged as a novel class of biotherapeutics, drawing significant attention in research and application. Creative Biolabs, a top preclinical CRO, is committed to providing efficient and precise ScFv-Fc recombinant antibody expression and purification services, helping our clients navigate the antibody drug development process with greater stability and success.
- Enhanced Affinity and Specificity: ScFv-Fc fusion antibodies maintain the high affinity of ScFv while benefiting from the stability and functionality provided by the Fc region. This makes ScFv-Fc particularly effective in targeted therapies and diagnostics, offering superior selectivity.
- Improved Biological Effects: The inclusion of the Fc region allows ScFv-Fc antibodies to more effectively trigger ADCC and CDC, thereby enhancing their therapeutic efficacy.
- Versatile Design and Application: The ScFv-Fc structure can be flexibly engineered to meet various research needs and application scenarios, making it widely applicable for treating diverse diseases such as cancer and immune disorders.
Creative Biolabs can swiftly engineer ScFv-Fc recombinant antibodies based on target sequences provided by clients. We will use multiple engineering techniques to enhance the antibody's properties, ensuring optimal expression levels and biological activity. Furthermore, we can conduct assays for biological activity, specificity analysis, and stability testing on the purified ScFv-Fc antibodies. We look forward to becoming your trusted partner. Please contact us to learn more about our scFv-Fc recombinant antibody expression and purification services.
- Ying, Binbin, et al. "High-Level Production of scFv-Fc Antibody Using an Artificial Promoter System with Transcriptional Positive Feedback Loop of Transactivator in CHO Cells." Cells 12.22 (2023): 2638.
- Parray, Hilal Ahmad, et al. "Identification of an anti-SARS-CoV-2 receptor-binding domain–directed human monoclonal antibody from a naïve semisynthetic library." Journal of Biological Chemistry 295.36 (2020): 12814-12821.
- Distributed under Open Access license CC BY 4.0, without modification.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

