Anti-Drug Antibody Products
Anti-drug antibodies (ADAs) represent immune reactions elicited against therapeutic agents. There are two primary categories of ADAs: non-neutralizing ADAs (bAbs), which bind to the drug specifically without impacting the interaction between the drug and its target, and neutralizing ADAs (nAbs), which block binding of the drug targets to the epitopes within or close to the active sites of the molecule or by causing conformational changes. ADAs have been identified in both preclinical and clinical studies, leading to notable alterations in toxicology, pharmacokinetics, and efficacy. Therefore, ADA-related detection and characterization assays have been developed, tailored, and optimized for each specific drug.
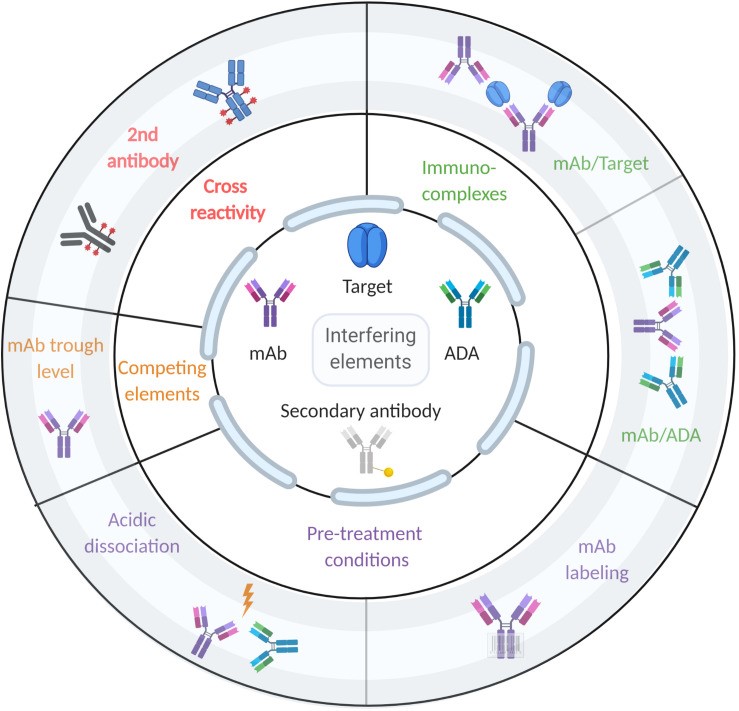 Fig. 1 illustrates the factors influencing ADA detection in immunoassays.1
Fig. 1 illustrates the factors influencing ADA detection in immunoassays.1
- Therapeutic Drug Monitoring (TDM)
Biopharmaceuticals, encompassing a diverse array of therapeutic agents such as monoclonal antibodies, fusion proteins, enzymes, hormones, interferons, growth factors, peptides, vaccines, oligonucleotides, and gene therapy products, have revolutionized the treatment landscape for numerous diseases. Anti-drug antibodies (ADAs) play a significant role in therapeutic drug monitoring (TDM), which is vital for tailoring drug doses to achieve optimal therapeutic concentrations swiftly while minimizing toxicity. TDM is a standard practice applied to selected small-molecule therapeutics, such as antibiotics, antiepileptics, immunosuppressants, and anticancer drugs, to monitor and adjust dosing for enhanced clinical outcomes.
- Positive Control of ADA Assay
Anti-drug antibody (ADA) assays are critical tools for assessing the immune response to therapeutic agents, including monoclonal antibodies, proteins, and other biologics, to ensure their efficacy and safety. To maintain the reliability and sensitivity of these assays, positive control antibodies are developed and utilized as essential reference materials. These controls help establish standard curves and define the assay's dynamic range, facilitating the accurate quantification of ADA levels. This process is particularly crucial in multi-center clinical trials, where it is imperative that different laboratories conducting ADA assays adhere to consistent standards to reliably assess a drug's safety and efficacy throughout its development.
Our Anti-Drug Antibody Products
Creative Biolabs offers specialized services for the custom development of anti-drug antibodies against biotech drugs or small molecule drugs, supporting drug monitoring and biosimilar development. Our expertise in managing immunization schedules, adjuvant selection, and methodologies for antibody screening and purification enables us to produce high-quality antibody reagents with increased success rates, even against challenging antigens such as oligonucleotide drugs and small molecules. Our anti-drug antibodies are ideally suited for use in pharmacokinetic (PK) bridging ELISAs and as reference standards in anti-drug antibody (ADA) assays. Our ready-made anti-drug antibodies come with data for PK, ADA, and inhibition assays, and are available in bulk, tailored to meet the immediate requirements of our clients. For inquiries related to anti-drug antibodies, please contact us.
- Vaisman-Mentesh, Anna, et al. "The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies." Frontiers in immunology 11 (2020): 1951. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Not For Clinical Use.