mTOR Complexes
Product List
 Loading...
Loading...- AbPlus™ Anti-MLST8 Magnetic Beads (VS-0724-YC593) (VS-0724-YC593)
-
- Target: MLST8
- Target Species: Human
- Application: IP, Protein Purification
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB
-
- Type: Rabbit IgG
- Application: WB, IHC-P
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, IP, FC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IP
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-P, IP, FC
-
- Species Reactivity: Human, Rat
- Type: Rabbit IgG
- Application: WB, IP
- Recombinant Mouse Anti-MTOR Antibody (VS-0923-FY112) (VS-0923-FY112)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-P
- Rabbit Anti-MTOR Polyclonal Antibody (VS3-WK1564)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ELISA
- Mouse Anti-AKT1S1 Recombinant Antibody (VS3-WK1098) (VS3-WK1098)
-
- Derivation: Mouse
- Species Reactivity: Human, Mouse
- Type: Mouse IgG1, κ
- Application: WB
- Mouse Anti-RPTOR Recombinant Antibody (VS3-WK1058) (VS3-WK1058)
-
- Derivation: Mouse
- Species Reactivity: Human, Mouse, Rat
- Type: Mouse IgG1, κ
- Application: WB, IHC-P
- Mouse Anti-RPTOR Recombinant Antibody (clone 5A3A4) (VS3-XY1390)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IHC, ICC
- Mouse Anti-RPTOR Recombinant Antibody (clone 6G9C4) (VS3-XY1389)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB
- Mouse Anti-RICTOR Recombinant Antibody (clone 7B3) (VS3-XY1372)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB, FC, IHC, ICC
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC, IP
-
- Derivation: Mouse
- Species Reactivity: Human, Mouse, Monkey
- Type: Mouse IgG1
- Application: WB, ELISA
-
- Derivation: Mouse
- Species Reactivity: Human, Mouse, Rat
- Type: Mouse IgG1, κ
- Application: WB, IHC
-
- Derivation: Mouse
- Species Reactivity: Human, Monkey, Mouse
- Type: Mouse IgG1
- Application: WB, IHC, ICC, FC, ELISA
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, WB, IHC, IF
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, WB, IF
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, IHC
-
- Species Reactivity: Human, Mouse, Monkey
- Type: Mouse IgG
- Application: WB, ELISA
- Rabbit Anti-RPTOR Polyclonal Antibody (MRO-2152-CN) (MRO-2152-CN)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IF, IHC, FC
- Rabbit Anti-MTOR Polyclonal Antibody (MRO-2048-CN) (MRO-2048-CN)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: IF, IHC, WB
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC, IP
- Mouse Anti-AKT1S1 Recombinant Antibody (clone 84Q32) (MOB-1680CT)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IP, WB
- Recombinant Mouse Anti-Human RICTOR Antibody (MOB-1650MZ)
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: ICC, IF, IHC-P, WB
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: ICC, IF
- Recombinant Anti-Human MLST8 Antibody (MOB-2706z)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: IgG
- Application: WB, IHC, FuncS
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, ICC, IF, IHC
- Rabbit Anti-NHP RICTOR Recombinant Antibody (VS-1024-XY448) (VS-1024-XY448)
-
- Species Reactivity: Human, Mouse, Non-human primate, Rat
- Type: Rabbit IgG
- Application: WB
- Rabbit Anti-NHP MLST8 Recombinant Antibody (VS-1024-XY214) (VS-1024-XY214)
-
- Species Reactivity: Human, Mouse, Non-human primate, Rat
- Type: Rabbit IgG
- Application: WB, IF, IP
- Recombinant Anti-Human MLST8 Antibody scFv Fragment (MOB-2706z-S(P))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: scFv
- Application: ELISA, WB, IP, FuncS
- Recombinant Anti-Human MLST8 Antibody Fab Fragment (MOB-2706z-F(E))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Fab
- Application: RIA, IF, FuncS
- Anti-Human RICTOR Immunohistochemistry Kit (VS-0525-XY6124)
-
- Species Reactivity: Human
- Target: RICTOR
- Application: IHC
- Anti-Human MTOR Immunohistochemistry Kit (VS-0525-XY4568)
-
- Species Reactivity: Human
- Target: MTOR
- Application: IHC
- Mouse Anti-MAPKAP1 Recombinant Antibody (clone 2506W54) (VS-0625-FY41)
-
- Species Reactivity: Human
- Type: Mouse IgG2a
- Application: WB, ELISA
- Anti-Mouse RPTOR Immunohistochemistry Kit (VS-0525-XY6240)
-
- Species Reactivity: Human, Mouse, Rat
- Target: RPTOR
- Application: IHC
- Anti-RPTOR Immunohistochemistry Kit (VS-0525-XY6239)
-
- Species Reactivity: Human
- Target: RPTOR
- Application: IHC
- Anti-Rat RICTOR Immunohistochemistry Kit (VS-0525-XY6125)
-
- Species Reactivity: Human, Rat
- Target: RICTOR
- Application: IHC
- Anti-Mouse MTOR Immunohistochemistry Kit (VS-0525-XY4569)
-
- Species Reactivity: Human, Mouse
- Target: MTOR
- Application: IHC
- Anti-Mouse AKT1S1 Immunohistochemistry Kit (VS-0525-XY283)
-
- Species Reactivity: Human, Mouse
- Target: AKT1S1
- Application: IHC
- Anti-AKT1S1 Immunohistochemistry Kit (VS-0525-XY282)
-
- Species Reactivity: Human
- Target: AKT1S1
- Application: IHC
- Anti-RICTOR Immunohistochemistry Kit (VS-0325-XY1921)
-
- Species Reactivity: Human, Mouse, Rat
- Target: RICTOR
- Application: IHC
- Mouse Anti-RICTOR Monoclonal Antibody (VS7-0425-WR781) (VS7-0425-WR781)
-
- Species Reactivity: Human, Mouse, Monkey
- Type: Mouse IgG
- Application: WB, IHC-P, IF, ICC, FC, ELISA
- Mouse Anti-mTOR Recombinant Antibody (clone 5E4D8) (VS7-0425-WR435)
-
- Species Reactivity: Human
- Type: Mouse IgG2a
- Application: WB, ELISA
- Mouse Anti-mTOR Recombinant Antibody (clone 8H5A5) (VS7-0425-WR434)
-
- Species Reactivity: Human
- Type: Mouse IgG2a
- Application: IHC
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, FC
- Rabbit Anti-MTOR Recombinant Antibody (VS13-YC747) (VS13-YC747)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
- Anti-MTOR Immunohistochemistry Kit (VS-0325-XY1390)
-
- Species Reactivity: Human, Mouse, Rat
- Target: MTOR
- Application: IHC
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: FCM
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IHC, ICC
-
- Antibody Host: Mouse
- Antibody Reactivity: Human
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IF, ICC, FC
-
- Species Reactivity: Human
- Type: IgG
- Application: WB, IF, ICC, FC
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IF, ICC
-
- Derivation: Phage display library screening
- Species Reactivity: Mouse, Rat, Human
- Type: IgG
- Application: WB, IP, FC, ICC, IF, IHC-P
-
- Derivation: Phage display library screening
- Species Reactivity: Human
- Type: IgG
- Application: ICC, IF, IHC-P, WB
-
- Derivation: Phage display library screening
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IP
- Chicken Anti-GBL Polyclonal IgY (BRD-0219MZ)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Chicken antibody
- Application: WB
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
The mechanistic target of rapamycin (mTOR) is a critical kinase in cellular growth and metabolism regulation, functioning through two distinct complexes known as mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2). These complexes play pivotal roles in integrating inputs from various upstream signals, including nutrients, growth factors, and cellular energy levels, to control downstream processes affecting cell growth, survival, and metabolism.
mTOR Complex 1 (mTORC1) is the more extensively studied of the two and is sensitive to rapamycin, an immunosuppressive drug from which mTOR gets its name. mTORC1 regulates protein synthesis, a fundamental aspect of cell growth, by phosphorylating S6 kinase (S6K) and the eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1). These phosphorylations lead to an increase in translation of specific mRNAs, particularly those encoding components of the protein synthesis machinery and metabolic enzymes. mTORC1 is also involved in lipid synthesis, autophagy inhibition, and lysosome biogenesis. It responds to nutrient availability (particularly amino acids), energy status (via AMPK), and growth factors (via the PI3K/Akt pathway).
mTOR Complex 2 (mTORC2), which is not sensitive to short-term treatment with rapamycin, has a role in regulating the cytoskeleton and activating Akt by phosphorylating it at a key serine residue (Ser473). Akt, in turn, is a critical mediator of growth factor signaling, promoting survival and growth. mTORC2 also influences ion transport and cellular metabolism and has been implicated in the regulation of glucose and lipid metabolism through different substrates.
Both complexes are composed of the mTOR kinase itself, but they have distinct regulatory and associated subunits that determine their substrate specificity and localization. For mTORC1, regulatory-associated protein of mTOR (RAPTOR) is a defining component, which helps recruit substrates for mTORC1. In contrast, rapamycin-insensitive companion of mTOR (RICTOR) is essential for mTORC2 activity and substrate specificity. The regulation of mTORC1 and mTORC2 involves multiple feedback loops and cross-talk with other signaling pathways, which adds complexity to their roles in cellular physiology. For example, prolonged activation of mTORC1 can lead to the inhibition of PI3K/Akt signaling through S6K-mediated phosphorylation and inhibition of insulin receptor substrate (IRS), which can contribute to insulin resistance in peripheral tissues.
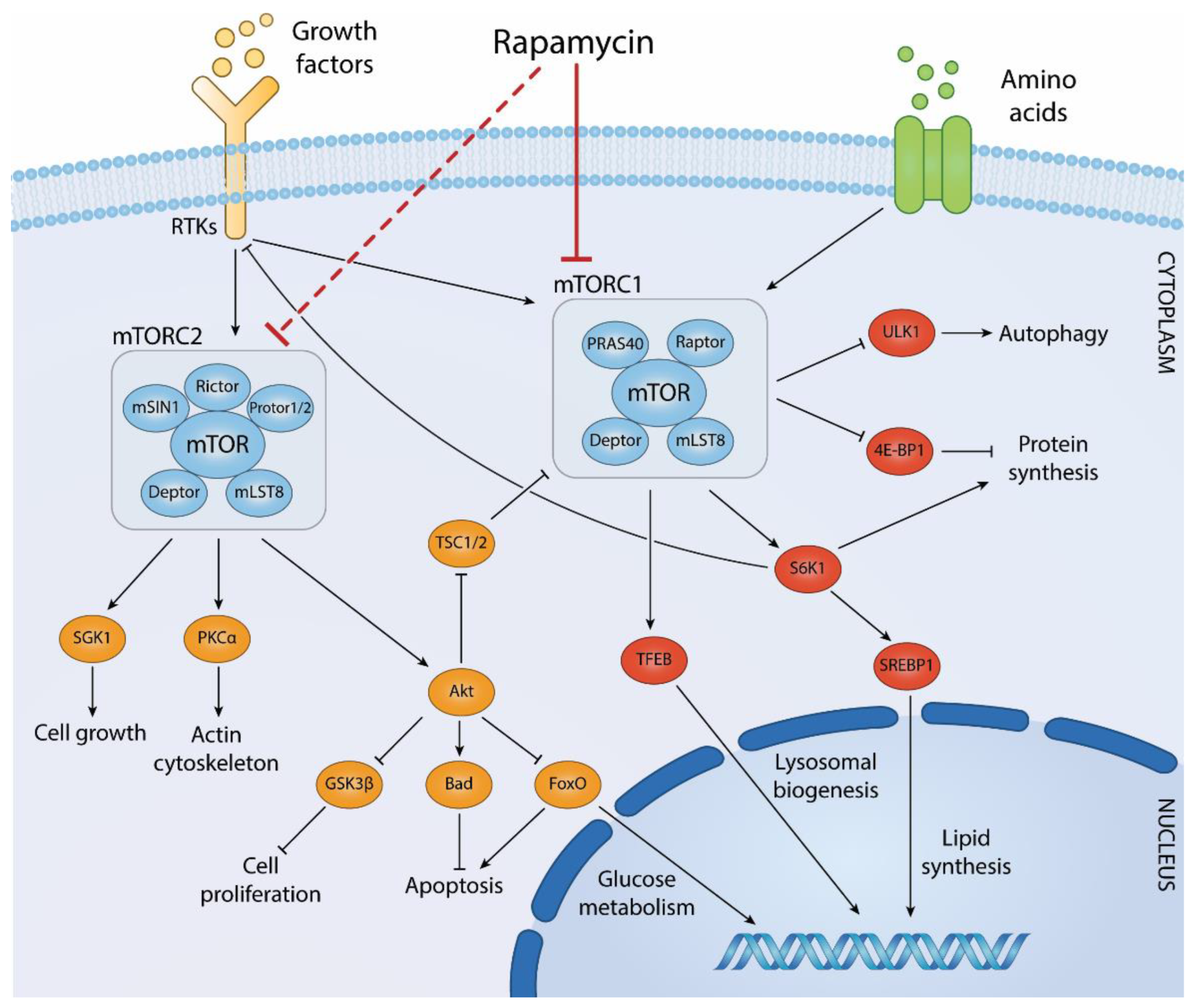 Figure 1 Biological processes regulated by mTOR complexes and downstream effector pathways. (Centonze, 2022)
Figure 1 Biological processes regulated by mTOR complexes and downstream effector pathways. (Centonze, 2022)
Representative Targets of mTOR Complexes
mTOR
mTOR is a highly conserved serine/threonine kinase that serves as a central regulator of cellular metabolism, growth, and survival. This protein is a core component of two distinct complexes: mTORC1 and mTORC2, each of which has unique substrates and biological functions. mTORC1 is sensitive to rapamycin and primarily regulates protein synthesis, cell growth, autophagy, and metabolism through its ability to sense and integrate diverse nutritional and environmental cues, including growth factors, energy status, oxygen levels, and amino acid availability. In contrast, mTORC2, which is less sensitive to rapamycin, plays a key role in regulating the cytoskeleton, cell survival, and lipid metabolism. The mTOR pathway is pivotal for its role in promoting anabolic processes such as lipid and protein synthesis while inhibiting catabolic processes like autophagy, making it crucial for cell growth and proliferation. Dysregulation of mTOR signaling is linked to a variety of diseases, including cancer, obesity, type 2 diabetes, and neurodegenerative disorders. In cancer, for example, hyperactivation of mTOR can drive tumor growth and resistance to therapy by enhancing protein synthesis and cell proliferation. Conversely, inhibition of mTOR has been shown to extend lifespan and reduce symptoms in various models of age-related disease.



RPTOR
RPTOR, known as regulatory-associated protein of MTOR, is a component of the mTORC1. RPTOR serves as a scaffolding protein that helps assemble the mTORC1 complex and mediates its interaction with substrates and other regulatory proteins, ensuring the effective transduction of signaling pathways essential for protein synthesis, lipid metabolism, and autophagy. The importance of RPTOR in the mTORC1 pathway lies in its ability to regulate the kinase activity of mTOR based on environmental cues. For instance, when nutrients are abundant, RPTOR assists mTORC1 in promoting anabolic processes, such as protein and lipid synthesis, while inhibiting catabolic processes like autophagy. This regulatory mechanism is vital for cellular and organismal growth and plays a role in maintaining energy homeostasis. Dysregulation of RPTOR and the mTORC1 pathway is associated with a range of diseases, including cancer, obesity, type 2 diabetes, and neurodegenerative disorders. In cancer, for example, overactivation of mTORC1 due to mutations or alterations in RPTOR can lead to unchecked cellular proliferation and survival, contributing to tumor growth and resistance to conventional therapies. Similarly, in metabolic diseases, impaired regulation of mTORC1 is linked to insulin resistance and altered lipid metabolism.
RICTOR
RICTOR, short for RPTOR independent companion of MTOR, complex 2, is an essential component of the mTOR Complex 2 (mTORC2). RICTOR is critical for the assembly and function of mTORC2, facilitating its ability to respond to growth factors and other extracellular signals. RICTOR helps mTORC2 phosphorylate a range of AGC kinase family members, including AKT, SGK1, and PKC, which are pivotal for cellular processes such as cell survival, migration, and differentiation. The phosphorylation of AKT by mTORC2 at Ser473, for example, is a crucial step in activating AKT signaling, which promotes cell growth and survival, highlighting RICTOR's role in vital signaling pathways. Importantly, the function of RICTOR and mTORC2 is implicated in several human diseases, particularly cancer. Overexpression or hyperactivation of RICTOR has been observed in various cancers, leading to enhanced AKT signaling and promoting oncogenic processes such as tumor growth and metastasis. Consequently, RICTOR has emerged as a potential therapeutic target, with research focused on developing inhibitors that can selectively disrupt mTORC2 activity to suppress cancer progression. In addition to cancer, the role of RICTOR in insulin signaling and lipid metabolism links it to metabolic diseases, such as diabetes and obesity. This makes it a subject of interest not only for cancer therapy but also for the treatment of metabolic disorders.
Full List of Targets of mTOR Complexes
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| AKT1S1 | Lobe; PRAS40 | 84335 | Q96B36 | AKT1S1 is a proline-rich substrate of AKT (MIM 164730) that binds 14-3-3 protein (see YWHAH, MIM 113508) when phosphorylated. |
| MLST8 | GBL; LST8; POP3; WAT1; GbetaL | 64223 | Q9BVC4 | |
| MTOR | Mechanistic Target Of Rapamycin Kinase; Rapamycin And FKBP12 Target 1; Mammalian Target Of Rapamycin; FK506-Binding Protein 12-Rapamycin Complex-Associated Protein 1; Mechanistic Target Of Rapamycin (Serine/Threonine Kinase); FK506 Binding Protein 12-Rapamycin Associated Protein 2; FKBP12-Rapamycin Complex-Associated Protein 1; Rapamycin Associated Protein FRAP2; FKBP-Rapamycin Associated Protein; Mechanistic Target Of Rapamycin; Rapamycin Target Protein 1; FRAP1; FRAP2 | 2475 | P42345 | The protein encoded by this gene belongs to a family of phosphatidylinositol kinase-related kinases. These kinases mediate cellular responses to stresses such as DNA damage and nutrient deprivation. This protein acts as the target for the cell-cycle arrest and immunosuppressive effects of the FKBP12-rapamycin complex. The ANGPTL7 gene is located in an intron of this gene. |
| RICTOR | RPTOR Independent Companion Of MTOR Complex 2; Rapamycin-Insensitive Companion Of MTOR; AVO3 Homolog; Pianissimo; HAVO3; RPTOR Independent Companion Of MTOR, Complex 2 | 253260 | Q6R327 | RICTOR and MTOR (FRAP1; MIM 601231) are components of a protein complex that integrates nutrient- and growth factor-derived signals to regulate cell growth. |
| RPTOR | Regulatory Associated Protein Of MTOR Complex 1; Raptor; P150 Target Of Rapamycin (TOR)-Scaffold Protein Containing WD-Repeats; Regulatory Associated Protein Of MTOR; Complex 1; P150 Target Of Rapamycin (TOR)-Scaffold Protein; Regulatory Associated Protein Of MTOR | 57521 | Q8N122 | This gene encodes a component of a signaling pathway that regulates cell growth in response to nutrient and insulin levels. The encoded protein forms a stoichiometric complex with the mTOR kinase, and also associates with eukaryotic initiation factor 4E-binding protein-1 and ribosomal protein S6 kinase. The protein positively regulates the downstream effector ribosomal protein S6 kinase, and negatively regulates the mTOR kinase. Multiple transcript variants encoding different isoforms have been found for this gene. |
Tested Data-Supported Products for Targeting mTOR Complexes
| CAT | Product Name | Biomarker | Assay | Image |
| ZG-069R | Mouse Anti-RICTOR Recombinant Antibody (ZG-069R) | RICTOR | WB |

|
| ZG-0084U | Rabbit Anti-RPTOR Recombinant Antibody (clone 1B10) | RPTOR | IHC |

|
| ZG-0473U | Rabbit Anti-Phospho-MTOR (S2481) Recombinant Antibody (clone 3H11) | MTOR | WB |

|
| ZG-0474U | Rabbit Anti-Phospho-MTOR (S2448) Recombinant Antibody (clone 1G6) | MTOR | WB |

|
| VS3-FY973 | Recombinant Rabbit Anti-MTOR (phospho Ser2448) Antibody (clone R08-7G7) | MTOR | WB |

|
- Centonze, Giorgia, et al. "ROCK ‘n TOR: an Outlook on keratinocyte Stem Cell Expansion in Regenerative Medicine via protein kinase Inhibition." Cells 11.7 (2022): 1130. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only. Not For Clinical Use.

