Apoptosis Extracellular Signals
Apoptosis, or programmed cell death, is a vital process that regulates cellular health and development by eliminating damaged, superfluous, or potentially harmful cells. This controlled cell elimination is triggered by various extracellular signals, including death ligands like tumor necrosis factor (TNF), Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL). These ligands interact with their respective death receptors on the cell surface, initiating a signaling cascade that activates caspase enzymes, leading to the execution of apoptosis. This pathway ensures that cells respond appropriately to external stimuli and stresses, maintaining tissue integrity and preventing uncontrolled cell proliferation.
In addition to direct signaling through death receptors, apoptosis can be triggered by environmental changes such as growth factor withdrawal or hormonal imbalances. For example, the absence of essential growth factors can disrupt mitochondrial function, leading to the release of pro-apoptotic factors like cytochrome c into the cytoplasm and initiating the intrinsic apoptotic pathway. Similarly, hormones like glucocorticoids can induce apoptosis in specific cells, such as lymphocytes, aiding in the regulation of the immune response and adaptation to physiological stresses.
Moreover, apoptosis is influenced by interactions with the extracellular matrix (ECM). Anoikis, a form of apoptosis triggered by cell detachment from the ECM, prevents detached cells from proliferating inappropriately, which is crucial for preventing cancer metastasis. The precise regulation of apoptosis by extracellular signals is critical for health and disease management, highlighting potential therapeutic targets in treating conditions where apoptosis is dysregulated, such as in autoimmune diseases, neurodegeneration, and various cancers.
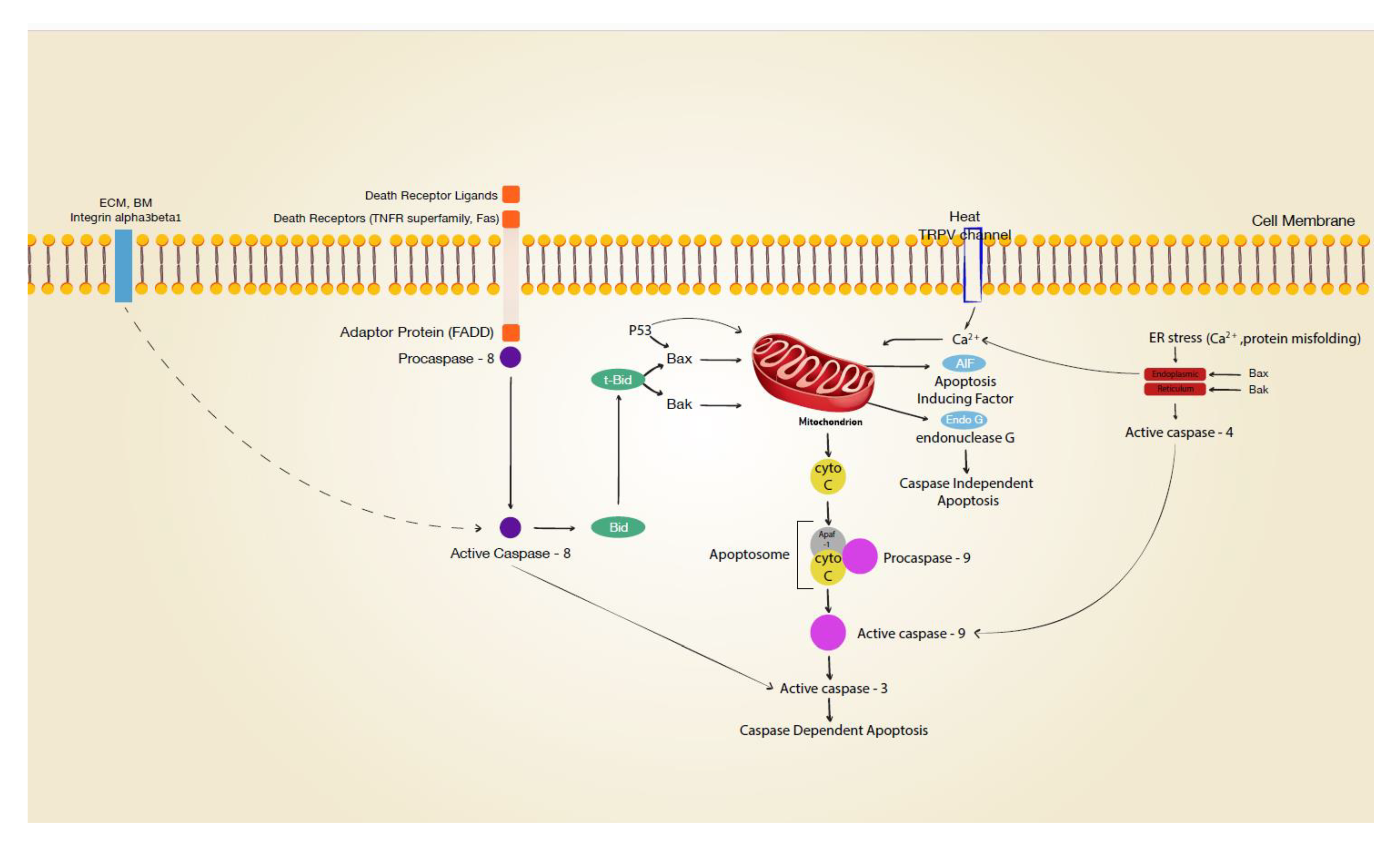 Figure 1 Signal transduction leading to apoptosis. (Rennekampff, 2021)
Figure 1 Signal transduction leading to apoptosis. (Rennekampff, 2021)
Representative Targets in Apoptosis Extracellular Signals
FASLG
FASLG, commonly known as Fas Ligand, is a type-II transmembrane protein that belongs to the tumor necrosis factor (TNF) family and plays a pivotal role in the regulation of the immune system and induction of apoptosis. FASLG binds to its receptor, Fas (also known as CD95), which is expressed on various cell types, including lymphocytes. This binding triggers a cascade of events leading to the formation of the death-inducing signaling complex (DISC) and subsequent activation of caspases, which are critical enzymes in the execution phase of apoptosis. The FAS/FASLG system is crucial for the maintenance of immune system homeostasis and the prevention of autoimmune diseases. It helps regulate the immune response by inducing the death of activated immune cells, thus preventing them from becoming autoreactive. FASLG also plays a role in immune privilege, a mechanism that protects certain sites of the body (like the eye and brain) from immune system attack, by inducing apoptosis in invading immune cells. Dysregulation of FASLG has been implicated in a variety of disorders. Inadequate expression can lead to immune system disorders, where the body fails to properly eliminate autoreactive or overactive immune cells, potentially resulting in autoimmune diseases. Conversely, excessive expression of FASLG has been associated with the destruction of beneficial cells, contributing to conditions like AIDS, where an excessive immune response leads to the depletion of T-cells.


GZMB
GZMB, or Granzyme B, is a serine protease released by cytotoxic T lymphocytes and natural killer (NK) cells, key components of the immune system responsible for the destruction of virally infected cells and tumor cells. Granzyme B is stored within the granules of these cytotoxic cells and is released during an immune response, usually in conjunction with the protein perforin, which facilitates the entry of granzyme B into target cells. Once inside the target cell, Granzyme B initiates apoptosis, a programmed cell death process, by cleaving and activating caspases, particularly caspase-3. This cascade leads to rapid cell death, effectively removing cells that are dangerous or no longer needed by the body. Additionally, Granzyme B can directly cleave other cellular substrates, contributing to apoptosis through various pathways, including the disruption of mitochondrial integrity and the activation of DNA damage responses. The role of Granzyme B in immune surveillance and defense makes it a critical factor in controlling viral infections and the proliferation of cancer cells. However, dysregulation of Granzyme B activity can lead to pathological conditions. For instance, excessive or misdirected activity of Granzyme B is implicated in some autoimmune diseases, where it may contribute to the destruction of healthy cells and tissues. Conversely, insufficient Granzyme B activity can result in inadequate immune responses, allowing persistent infections and cancer cells to evade immune detection.

TIMP3
TIMP3, or Tissue Inhibitor of Metalloproteinases 3, is one of the four members of the TIMP family that regulates the activity of matrix metalloproteinases (MMPs). TIMP3 is unique among the TIMPs in that it is tightly bound to the extracellular matrix (ECM) and inhibits not only MMPs but also a disintegrin and metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) families of enzymes. These proteases are involved in the breakdown of the extracellular matrix, which is crucial for normal tissue remodeling, repair, and angiogenesis. TIMP3 plays a critical role in maintaining tissue homeostasis by inhibiting the excessive proteolytic activity of MMPs, ADAMs, and ADAMTSs. By regulating these enzymes, TIMP3 helps control processes such as ECM degradation, which is critical during wound healing and in the maintenance of tissue structure. Dysregulation of TIMP3 expression and function has been linked to a variety of pathological conditions including cardiovascular diseases, such as atherosclerosis and heart failure, where imbalance in ECM remodeling contributes to disease progression. In addition to its roles in cardiovascular health, TIMP3 is also significant in the pathogenesis of cancer and ocular diseases. In cancer, decreased levels of TIMP3 can lead to increased tumor growth and metastasis due to enhanced ECM degradation and altered cell migration. Conversely, in ocular diseases like age-related macular degeneration (AMD), overexpression of TIMP3 is thought to contribute to abnormal extracellular matrix accumulation and choroidal neovascularization.
Full List of Targets in Apoptosis Extracellular Signals
| Biomarker | Alternative Names | Gene ID | UniProt ID | Roles |
| FASLG | Fas Ligand; Fas Ligand (TNF Superfamily, Member 6); Apoptosis Antigen Ligand; Fas Antigen Ligand; CD95 Ligand; APT1LG1; TNFSF6; CD95-L; CD95L; APTL; FASL | 356 | P48023 | This gene is a member of the tumor necrosis factor superfamily. The primary function of the encoded transmembrane protein is the induction of apoptosis triggered by binding to FAS. The FAS/FASLG signaling pathway is essential for immune system regulation, including activation-induced cell death (AICD) of T cells and cytotoxic T lymphocyte induced cell death. It has also been implicated in the progression of several cancers. Defects in this gene may be related to some cases of systemic lupus erythematosus (SLE). Alternatively spliced transcript variants have been described. |
| GZMA | GZMA; Hanukah Factor Serine Protease); EC 3.4.21.78; Cytotoxic T-Lymphocyte Proteinase 1; Granzyme A (Cytotoxic T-Lymphocyte-Associated Serine Esterase-3; Hanukah Factor Serine Protease); CTL Tryptase; EC 3.4.21; Granzyme-1; Hanukkah Factor; CTLA3; Fragme | 3001 | P12544 | Cytolytic T lymphocytes (CTL) and natural killer (NK) cells share the remarkable ability to recognize, bind, and lyse specific target cells. They are thought to protect their host by lysing cells bearing on their surface 'nonself' antigens, usually peptides or proteins resulting from infection by intracellular pathogens. The protein described here is a T cell- and natural killer cell-specific serine protease that may function as a common component necessary for lysis of target cells by cytotoxic T lymphocytes and natural killer cells. |
| GZMB | C11; HLP; CCPI; CGL1; CSPB; SECT; CGL-1; CSP-B; CTLA1; CTSGL1 | 3002 | P10144 | This gene encodes a member of the granzyme subfamily of proteins, part of the peptidase S1 family of serine proteases. The encoded preproprotein is secreted by natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) and proteolytically processed to generate the active protease, which induces target cell apoptosis. This protein also processes cytokines and degrades extracellular matrix proteins, and these roles are implicated in chronic inflammation and wound healing. Expression of this gene may be elevated in human patients with cardiac fibrosis. |
| GZMH | GZMH; Granzyme H; Cytotoxin Serine Protease-C; Cathepsin G-Like 2; CTSGL2; EC 3.4.21.-; Cytotoxic T-Lymphocyte Proteinase; CTLA1; Granzyme H (Cathepsin G-Like 2, Protein H-CCPX); Cytotoxic T-Lymphocyte-Associated Serine Esterase 1 | 2999 | P20718 | Cytotoxic chymotrypsin-like serine protease with preference for bulky and aromatic residues at the P1 position and acidic residues at the P3 and P4 sites. Probably necessary for target cell lysis in cell-mediated immune responses. Participates in the antiviral response via direct cleavage of several proteins essential for viral replication. |
| SRGN | SRGN; serglycin; PRG, PRG1,proteoglycan 1, secretory granule; PPG; serglycin proteoglycan; Chondroitin sulfate proteoglycan core protein; Cytolytic granule proteoglycan core protein; FLJ12930; gp600; Hematopoetic proteoglycan core protein; Mastocytoma proteoglycan core protein; MGC9289; OTTHUMP00000019716; P.PG; PG19 core protein; Pgsg; Platelet proteoglycan core protein; platelet proteoglycan protein core; PLATELET PROTEOGLYCAN PROTEIN CORE; PPG; PRG; PRG1; PROTEOGLYCAN 1; proteoglycan 1, secretory granule; Proteoglycan 10K core protein; Proteoglycan peptide core protein; PROTEOGLYCAN PROTEIN CORE FOR MAST CELL SECRETORY GRANULE; secretory granule proteoglycan 1; Secretory granule proteoglycan core protein; Serglycin; serglycin proteoglycan; Sgc; Srgn; SRGN_HUMAN; p.PG; OTTHUMP00000019716; proteoglycan 1, secretory granule; platelet proteoglycan core protein; hematopoetic proteoglycan core peptide; hematopoetic proteoglycan core protein; secretory granule proteoglycan core peptide; secreto; PRG; PRG1; | 5552 | P10124 | This gene encodes a protein best known as a hematopoietic cell granule proteoglycan. Proteoglycans stored in the secretory granules of many hematopoietic cells also contain a protease-resistant peptide core, which may be important for neutralizing hydrolytic enzymes. This encoded protein was found to be associated with the macromolecular complex of granzymes and perforin, which may serve as a mediator of granule-mediated apoptosis. Two transcript variants, only one of them protein-coding, have been found for this gene. [provided by RefSeq, Jul 2010] |
| TIMP3 | TIMP3; Human TIMP3 | 7078 | P35625 | |
| TNF-alpha | APC1 protein; Cachectin; Cachetin; DIF; TNF; TNF, monocyte-derived; TNFA; TNF-A; TNFalpha; TNF-alpha; TNF-alphacachectin; TNFATNF, macrophage-derived; TNFG1F; TNFSF1A; TNFSF2; TNFSF2TNF superfamily, member 2; tumor necrosis factor (TNF superfamily, member 2); tumor necrosis factor alpha; Tumor necrosis factor ligand superfamily member 2; tumor necrosis factor; tumor necrosis factor-alpha; TNF-a; tumor necrosis factor ligand 1F | 100009088 | P04924 | Tumor necrosis factor (TNF-alpha ), also known as cachectin and TNFSF2, is the prototypic ligand of the TNF superfamily and family. The 26 kDa type II transmembrane protein is assembled intracellularly to form a noncovalently linked homotrimer. Rabbit TNF-alpha is 235 amino acids (aa) in length and contains a 35 aa cytoplasmic domain, a 21 aa transmembrane region, and a 179 extracellular domain (ECD). TNF-alpha is produced by several lymphoid cells as well as by astrocytes, endothelial cells, and smooth muscle cells. |
| TNFSF10 | TL2; APO2L; CD253; TANCR; TRAIL; Apo-2L; TNLG6A | 8743 | P50591 | The protein encoded by this gene is a cytokine that belongs to the tumor necrosis factor (TNF) ligand family. This protein preferentially induces apoptosis in transformed and tumor cells, but does not appear to kill normal cells although it is expressed at a significant level in most normal tissues. This protein binds to several members of TNF receptor superfamily including TNFRSF10A/TRAILR1, TNFRSF10B/TRAILR2, TNFRSF10C/TRAILR3, TNFRSF10D/TRAILR4, and possibly also to TNFRSF11B/OPG. The activity of this protein may be modulated by binding to the decoy receptors TNFRSF10C/TRAILR3, TNFRSF10D/TRAILR4, and TNFRSF11B/OPG that cannot induce apoptosis. The binding of this protein to its receptors has been shown to trigger the activation of MAPK8/JNK, caspase 8, and caspase 3. Alternatively spliced transcript variants encoding different isoforms have been found for this gene. |
| TNFSF15 | TL1; VEGI | 9966 | O95150 | The protein encoded by this gene is a cytokine that belongs to the tumor necrosis factor (TNF) ligand family. This protein is abundantly expressed in endothelial cells, but is not expressed in either B or T cells. The expression of this protein is inducible by TNF and IL-1 alpha. This cytokine is a ligand for receptor TNFRSF25 and decoy receptor TNFRSF21/DR6. It can activate NF-kappaB and MAP kinases, and acts as an autocrine factor to induce apoptosis in endothelial cells. This cytokine is also found to inhibit endothelial cell proliferation, and thus may function as an angiogenesis inhibitor. Two transcript variants encoding different isoforms have been found for this gene. |
Tested Data-Supported Products for Targeting Apoptosis Extracellular Signals
| CAT | Product Name | Biomarker | Assay | Image |
| TAB-261CL | Anti-Human FASLG Recombinant Antibody | FASLG | WB |

|
| HPAB-0179CQ | Human Anti-FASLG Recombinant Antibody (clone 4G11) | FASLG | WB |

|
| ZG-0701J | Rabbit Anti-GZMB Recombinant Antibody (clone 6A1) | GZMB | IHC |

|
- Rennekampff, Hans-Oliver, and Ziyad Alharbi. "Burn injury: mechanisms of keratinocyte cell death." Medical Sciences 9.3 (2021): 51. Distributed under Open Access license CC BY 4.0, without modification.
 Loading...
Loading...- Human Anti-TNFSF10 Recombinant Antibody (clone DR4-4) (VS-0423-CJ41)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: IB, IA, FC
-
- Type: Mouse IgG1
- Application: ELISA, FC
- Mouse Anti-TNF-alpha Recombinant Antibody (VS-0322-LC34) (VS-0322-LC34)
-
- Species Reactivity: Rabbit, Cotton Rat, Feline, Guinea pig, Human, Mouse, Rat, Canine, Equine, Rhesus
- Application: WB
- Mouse Anti-TNF-alpha Recombinant Antibody (VS-0322-LC33) (VS-0322-LC33)
-
- Species Reactivity: Rabbit
- Application: Neut
- Human Anti-TNFSF10 Recombinant Antibody (HPAB-1451-FY) (HPAB-1451-FY)
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized IgG
- Application: Activ
- Mouse Anti-TNFSF10 Recombinant Antibody (clone CBL494) (NEUT-2177CQ)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IHC, Neut
- Mouse Anti-TNFSF10 Recombinant Antibody (clone CBL262) (NEUT-2176CQ)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: FC, Neut
- Mouse Anti-TNFSF10 Recombinant Antibody (clone 2E5) (NEUT-2174CQ)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: FuncS, FC, Neut
- Mouse Anti-TNFSF10 Recombinant Antibody (NEUT-2173CQ) (NEUT-2173CQ)
-
- Species Reactivity: Human
- Type: Mouse IgG1, κ
- Application: ELISA, Neut, WB
- Mouse Anti-TNFSF10 Recombinant Antibody (clone CBL186) (NEUT-2172CQ)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: FC, IHC, Neut, WB
- Rat Anti-Tnfsf10 Recombinant Antibody (clone N2B2) (NEUT-2171CQ)
-
- Species Reactivity: Mouse
- Type: Rat IgG2a, κ
- Application: FC, FuncS, Neut
- Mouse Anti-TNFSF10 Recombinant Antibody (clone RIK-2) (NEUT-2170CQ)
-
- Species Reactivity: Human
- Type: Mouse IgG1, κ
- Application: FC, FuncS, Neut
-
- Species Reactivity: Human
- Application: ELISA, WB
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: IHC-P, WB
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: WB
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: IHC-FoFr, IHC-P, WB
- Recombinant Anti-human TNFSF10 Antibody (MOB-987)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: IgG
- Application: ELISA, WB, FC, FuncS
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, IHC
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG2
- Application: WB
-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG
- Application: ELISA, WB, FC, FuncS
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: FC
- Recombinant Human Anti-human TNFSF10 Antibody Fab Fragment (MHH-987-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Fab
- Application: FC, RIA, Biosensors, FuncS
- Recombinant Human Anti-human TNFSF10 Antibody scFv Fragment (MHH-987-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: scFv
- Application: ELISA, IP, FuncS
- Human Anti-TNFSF10 Recombinant Antibody; Fab Fragment (HPAB-1451-FY-F(E)) (HPAB-1451-FY-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: Activ
- Human Anti-TNFSF10 Recombinant Antibody; scFv Fragment (HPAB-1451-FY-S(P)) (HPAB-1451-FY-S(P))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized scFv
- Application: Activ
- Mouse Anti-NHP TNFSF10 Recombinant Antibody (clone 124723) (VS-1024-XY511)
-
- Species Reactivity: Human, Non-human primate
- Type: Mouse IgG1
- Application: ELISA
- Recombinant Anti-human TNFSF10 Antibody Fab Fragment (MOB-987-F(E))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Fab
- Application: ELISA, FuncS
- Recombinant Anti-human TNFSF10 Antibody scFv Fragment (MOB-987-S(P))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: scFv
- Application: FC, Neut, Funcs
-
- Antibody Host: Mouse
- Antibody Reactivity: Human
- Human Anti-TNFSF10 (clone DR4-4) scFv-Fc Chimera (VS-0425-FY49)
-
- Species Reactivity: Human
- Type: Human IgG1, scFv-Fc
- Application: WB, ELISA, FC, Cyt
-
- Species Reactivity: Human
- Target: TNFSF10
- Host Animal: Human
- Application: ELISA, FC, Cell-uptake
- Anti-TIMP3 Immunohistochemistry Kit (VS-0525-XY7275)
-
- Species Reactivity: Human
- Target: TIMP3
- Application: IHC
- Anti-Human TIMP3 Immunohistochemistry Kit (VS-0525-XY7276)
-
- Species Reactivity: Human, Porcine
- Target: TIMP3
- Application: IHC
- Anti-TNFSF10 Immunohistochemistry Kit (VS-0525-XY7387)
-
- Species Reactivity: Human
- Target: TNFSF10
- Application: IHC
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human IgG
- Application: Activation
- Chicken Anti-TNFSF10 Polyclonal IgY (BRD-0598MZ)
-
- Species Reactivity: Human
- Type: Chicken antibody
- Application: WB
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
For Research Use Only. Not For Clinical Use.

