Plasmid Analytical Method Development & Validation Service
Background
In the development and manufacturing of plasmid DNA (pDNA) for therapeutic and vaccine applications, regulatory authorities increasingly demand comprehensive quality data supported by robust, validated analytical methods. Whether used as templates for mRNA production, raw materials for gene therapy, or as DNA vaccines themselves, plasmids must meet stringent quality attributes including identity, purity, potency, and safety. Analytical method development and validation form the cornerstone of this quality assessment, enabling manufacturers to ensure consistent product performance, meet regulatory expectations, and safeguard patient outcomes.
Core Services Offerings at Creative Biolabs
At Creative Biolabs, we offer specialized plasmid analytical method development and validation services tailored for plasmid-based biopharmaceuticals. With in-depth expertise in analytical sciences and regulatory compliance, our team delivers custom method development strategies that align with ICH Q2(R1) guidelines and current Good Manufacturing Practice (cGMP) requirements. Our services are designed to support your plasmid programs at all development stages—from research-grade constructs to GMP-grade production.
Identity Testing
- Restriction enzyme mapping
- PCR-based sequence confirmation
- DNA barcoding and NGS
Purity and Impurity Analysis
- Host cell genomic DNA quantification
- Host cell RNA content
- Endotoxin levels (LAL assay)
- Residual host cell proteins (HCPs)
- Residual antibiotics and detergents
- Plasmid topological forms (supercoiled, open circular, linear)
Potency Assays
- In vitro transfection efficiency
- Reporter gene expression quantification
Quantification and Concentration
- UV absorbance (A260/A280)
- qPCR/ddPCR-based quantification
- Fluorescence-based assays
Stability-Indicating Methods
- Degradation product detection under stress conditions
- Freeze-thaw and long-term stability assays
Our plasmid analytical method development and validation service is designed to support both R&D-stage and GMP-grade plasmid products. We offer method development and validation solutions that meet the requirements for various types of testing, including but not limited to:
All methods developed can be validated according to ICH Q2(R1) parameters including specificity, accuracy, precision, linearity, range, and robustness.
Featured Technologies
Our analytical platform integrates cutting-edge technologies to ensure precise, reproducible data:
- qPCR & Droplet Digital PCR (ddPCR): For sensitive quantification of plasmid copy number, residual DNA, and sequence-specific identity.
- 2D Gel Electrophoresis: Enables high-resolution separation and quantification of plasmid isoforms.
- High-Performance Liquid Chromatography (HPLC): Including anion exchange (AEX), reversed-phase (RP), and size-exclusion chromatography (SEC) to assess purity and integrity.
- Endotoxin Testing (LAL/KTA): Employs kinetic turbidimetric or chromogenic assays with stringent detection limits suitable for clinical-grade plasmid materials.
- Spectroscopic Analysis: UV and fluorescence-based tools for rapid plasmid concentration and purity estimation.
Service Workflow
We follow a streamlined, phase-appropriate workflow designed to ensure technical and regulatory robustness:
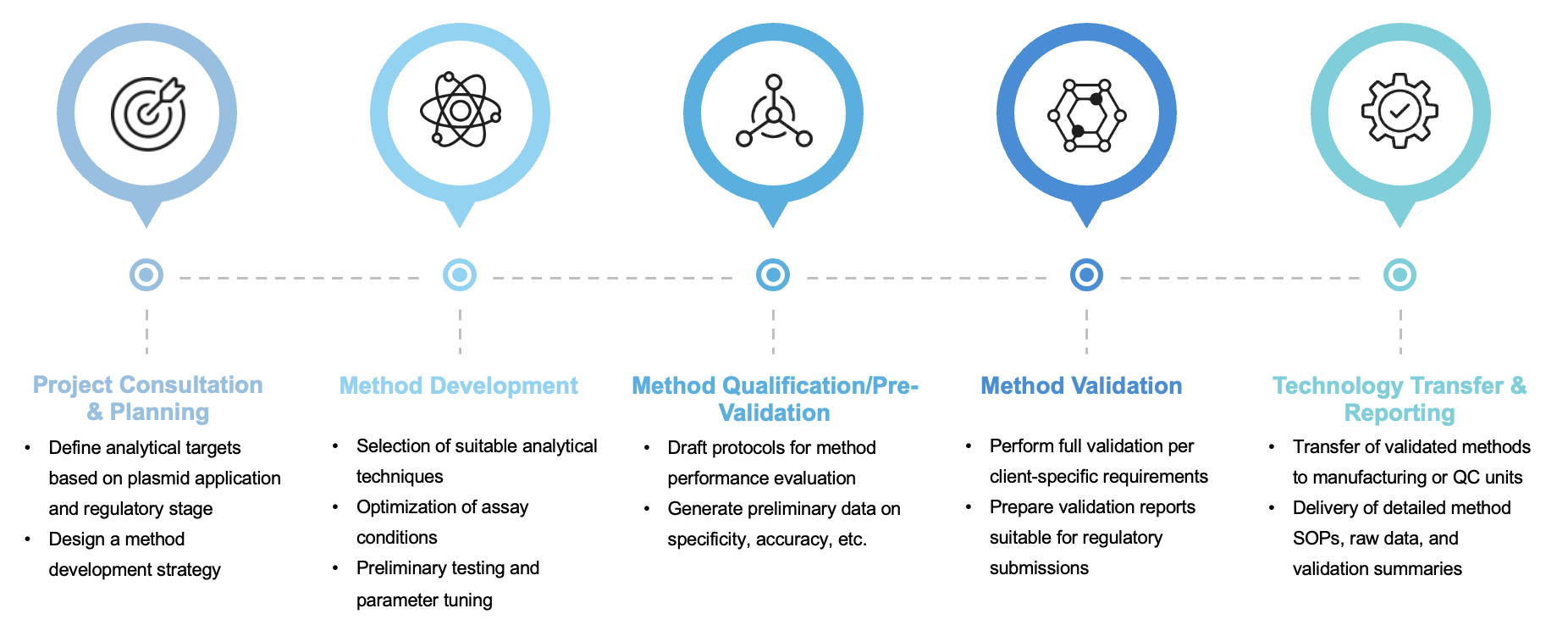
Why Choose Creative Biolabs
Customization Capability: We adapt analytical designs based on plasmid structure (e.g., minicircle, linear, episomal) and intended use.
Experienced Team: Our team includes Ph.D.-level scientists with decades of experience in nucleic acid analytics and method validation under QA oversight.
End-to-End Integration: Services can be bundled with our upstream plasmid production and QC testing platforms, reducing your outsourcing complexity.
Fast Turnaround: Efficient project execution timelines without compromising data integrity or regulatory standards.
Partner With Us
Collaborating with Creative Biolabs means gaining a dedicated partner equipped to navigate the complexities of plasmid analytics. We understand the challenges inherent to plasmid-based therapeutic development, from product heterogeneity to stringent release criteria. By integrating robust analytical methods early in your development pipeline, you can accelerate timelines, minimize regulatory risk, and ensure confidence in product quality.
We welcome you to contact our scientific team to discuss your plasmid quality testing and analytical validation needs. Let us help you build a solid foundation for your therapeutic plasmid program.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

