Antibody Characterization Service
Unlocking the Structural and Functional Complexity of Antibodies
The development of antibody-based therapeutics demands comprehensive characterization to ensure their identity, quality, and consistency. Given the inherent complexity of antibody molecules—including their heterogeneity, glycosylation patterns, and post-translational modifications—an accurate understanding of structural and functional attributes is essential. At Creative Biolabs, our antibody characterization service is designed to support every phase of therapeutic antibody development, from early discovery to late-stage product release, through rigorous, science-driven assessment strategies. Our multidisciplinary team leverages advanced technologies and extensive regulatory understanding to deliver highly reliable and tailored characterization solutions.
Comprehensive Profiling at Every Level
Our antibody characterization platform encompasses a broad range of analyses tailored to meet the exacting standards of biologics development. We focus on delivering detailed molecular insights that are critical for product qualification, batch-to-batch consistency, comparability studies, and regulatory submission. Our service portfolio includes:
- Primary Structure Determination: Amino acid sequencing, disulfide bond mapping, and identification of sequence variants.
- Higher-Order Structure (HOS) Assessment: Circular dichroism (CD), differential scanning calorimetry (DSC), and hydrogen-deuterium exchange (HDX) for secondary and tertiary structure evaluation.
- Glycosylation Profiling: Analysis of N- and O-linked glycans using LC-MS and HILIC-FLD platforms.
- Charge Variant and Isoform Analysis: Focused ion exchange chromatography and isoelectric focusing for charge heterogeneity mapping.
- Aggregate and Fragment Analysis: Size-exclusion chromatography and dynamic light scattering to evaluate molecular integrity and aggregation tendencies.
- Binding Affinity and Functionality Testing: Cell-based assays and surface-based technologies to examine antigen-binding capabilities and effector functions.
Each characterization study is custom-designed to fit the molecule's specific format—be it monoclonal antibody (mAb), bispecific construct, fusion protein, or ADC—and to align with the program's development stage and regulatory expectations.
Advanced Technologies Empowering Reliable Insights
We employ an integrated suite of high-end analytical technologies to dissect antibody attributes with precision and reproducibility. Our laboratories are equipped with:
- Mass Spectrometry Platforms: High-resolution Orbitrap and Q-TOF systems enable accurate mass measurement, peptide mapping, and impurity detection.
- Capillary Electrophoresis (CE): For size heterogeneity, purity, and charge variant profiling.
- Chromatography Systems (HPLC/UHPLC): Used in conjunction with UV, fluorescence, or MS detectors for profiling of structural variants.
- Bio-Layer Interferometry (BLI) and SPR: Quantitative kinetics and binding affinity measurements under physiologically relevant conditions.
- Spectroscopic Techniques (CD, FTIR): Non-destructive tools for evaluating protein folding and structural integrity.
- Imaging Techniques (EM, AFM): For morphology analysis and aggregate visualization.
Each platform is supported by rigorous data interpretation and detailed reporting that aligns with global regulatory expectations.
A Rational, Stepwise Workflow Tailored to Your Molecule
We follow a carefully structured yet flexible characterization workflow, designed to meet individual project goals with scientific robustness and operational transparency:
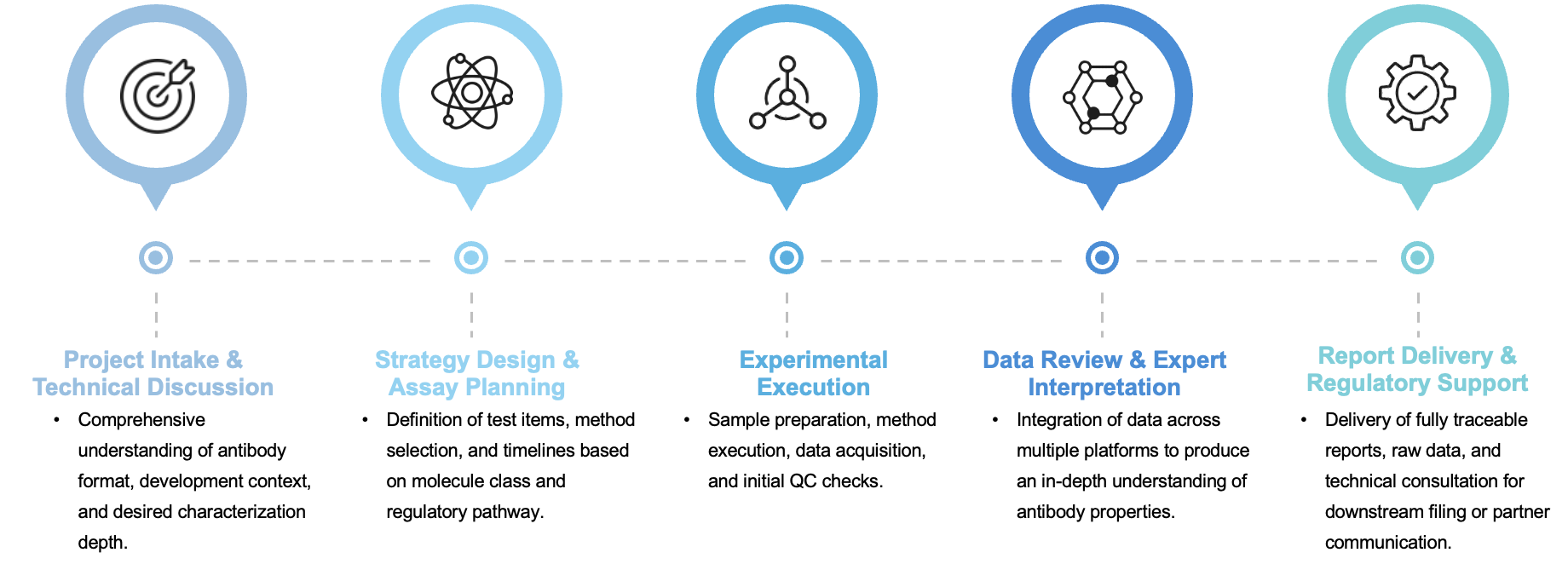
Why Our Characterization Platform Stands Out
Creative Biolabs provides more than technical execution—we deliver confidence in your therapeutic candidate. Key strengths of our Antibody Characterization Service include:
Molecule-Specific Expertise
Decades of experience with humanized, chimeric, and engineered antibodies, including Fc-modified and multispecific formats.
Integrated Multimodal Approach
Orthogonal testing strategies to ensure data cross-validation and analytical redundancy.
Scalable Support
From small-scale discovery samples to large-batch GMP characterization, our flexible service scales to your needs.
Customizability
No "one-size-fits-all" protocols—each project benefits from customized strategies aligned with its unique development timeline.
A Collaborative Partnership Rooted in Scientific Excellence
By choosing Creative Biolabs, you gain a partner dedicated to your antibody's success at every stage. We operate with transparency, scientific rigor, and commitment to timelines—ensuring you have dependable data to drive informed decisions. Our collaborative model fosters open communication and ongoing support, making us a trusted extension of your R&D and regulatory teams.
Let us help you decode the complexity of your antibody candidate. Contact us today to discuss your specific needs and experience the depth of expertise we bring to antibody characterization.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

