Antibody CMC Analytical Support Service
Empowering Regulatory Readiness Through Rigorous Analytical Strategy
Chemistry, Manufacturing, and Controls (CMC) development is a foundational element of any successful therapeutic antibody program. Regulatory agencies place increasing emphasis on comprehensive, phase-appropriate analytical support to define the identity, strength, purity, and potency of biologic products. At Creative Biolabs, we deliver tailored antibody CMC analytical support services that bridge scientific excellence with compliance precision, helping clients navigate every stage from IND-enabling studies to commercial launch with confidence.
Full-Spectrum Assessment Covering DS and DP Stages
Our service is designed to support the full CMC lifecycle with a focus on antibody products, including monoclonal antibodies (mAbs), bispecifics, antibody-drug conjugates (ADCs), Fc-fusion proteins, and novel recombinant formats.
Whether initiating early development or preparing a marketing application, our CMC analytical services are built to meet phase-specific demands while maintaining regulatory alignment. Core offerings include:
- Analytical Method Development & Qualification: Fit-for-purpose protocols for assessing identity, content, impurities, and biological activity.
- Release and Stability Testing Panels: Customized for Drug Substance (DS) and Drug Product (DP), including critical quality attributes (CQAs) and ICH-compliant stability protocols.
- Comparability & Biosimilarity Assessments: In-depth analyses to support manufacturing changes or biosimilar development through orthogonal techniques.
- ICH Q6B Characterization: Includes structural, physicochemical, biological, and immunochemical testing per international guidelines.
- Process-Related Impurity Profiling: Monitoring of residual host cell proteins (HCPs), DNA, Protein A, endotoxins, and other process-derived contaminants.
- Forced Degradation & Stress Testing: Supports degradation pathway elucidation and stability-indicating method validation.
These services are integrated seamlessly with regulatory expectations from FDA, EMA, PMDA, and NMPA, tailored to antibody-specific nuances.
Precision-Driven Techniques for Analytical Depth
Our CMC analytical portfolio leverages cutting-edge instrumentation and a deep understanding of biologic complexity. Core platforms include:
- High-Resolution Mass Spectrometry (HR-MS): Comprehensive primary sequence confirmation, post-translational modification profiling, and intact mass analysis.
- Chromatographic Assays (HPLC/UPLC): SEC, IEX, RP, and affinity-based assays for purity, heterogeneity, and fragmentation analysis.
- Capillary Electrophoresis (CE-SDS, icIEF): For charge variants, glycoform profiling, and molecular weight verification.
- Surface Plasmon Resonance (SPR) & BLI: High-throughput and kinetic binding affinity measurements.
- Cell-Based Bioassays & ELISA: Customizable potency and activity testing using validated functional endpoints.
- Subvisible and Visible Particle Analysis: For DP evaluation per USP <788> and <789>.
- Spectroscopic & Thermodynamic Techniques: Including CD, FTIR, and DSC for higher-order structure assessment.
Our method lifecycle management ensures traceability, reproducibility, and scalability across all stages of product development.
Structured Workflow for Regulatory Robustness
We offer a comprehensive project management framework that ensures consistent execution, risk mitigation, and documentation clarity throughout the CMC analytical process:
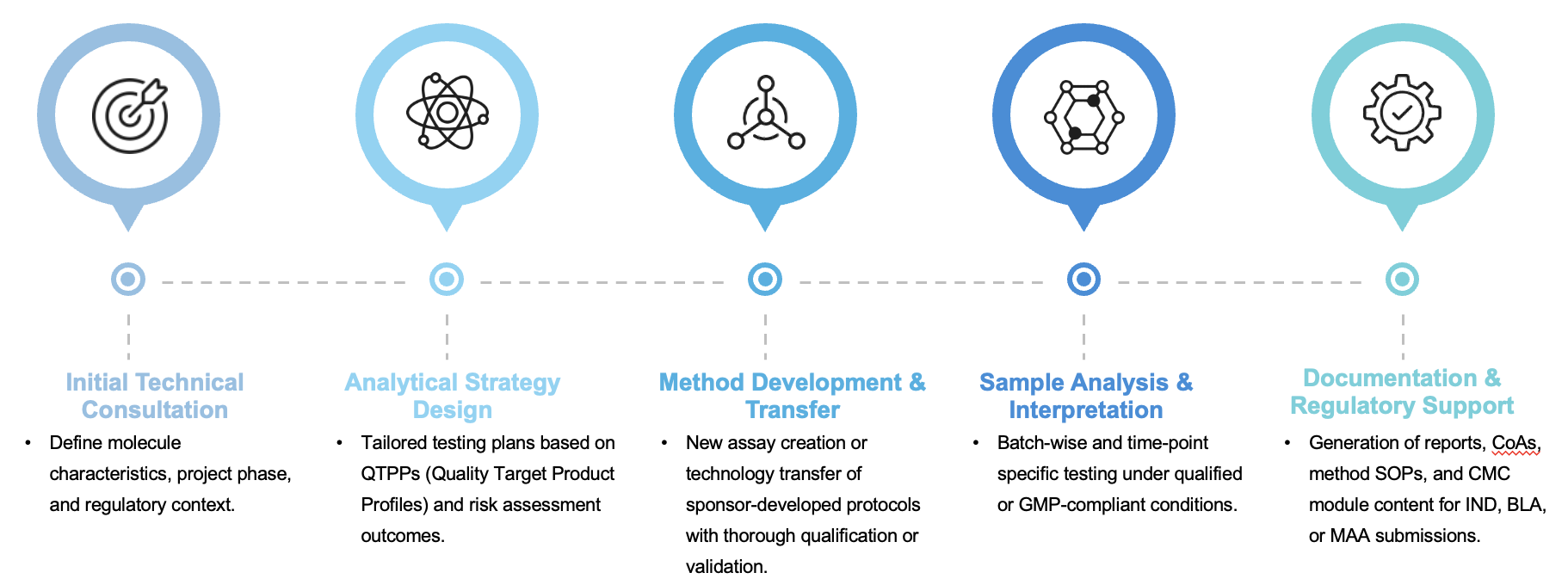
All studies are executed in alignment with current good manufacturing practices (cGMP) and ICH guidelines, ensuring global regulatory compatibility.
Why Choose Our CMC Analytical Services?
Our antibody CMC analytics offering is distinguished by its scientific rigor, operational agility, and regulatory foresight:
Antibody-Focused Expertise
Deep technical understanding of immunoglobulin biology and CQA-specific analytical requirements.
Seamless Phase Transition Support
Consistent analytical oversight from discovery to licensure, reducing development friction.
Customized Methodologies
Not limited to template protocols; every test panel is built with your antibody and process in mind.
Dual Facility Options
Choice of preclinical-grade or GMP-compliant testing laboratories depending on your project needs.
Expedited Timelines Available
Streamlined onboarding and rapid data turnaround for time-sensitive submissions.
Your Strategic Partner in Biologics Development
At Creative Biolabs, we understand that effective CMC analytical support extends beyond the bench. It's about enabling data-driven decision-making, facilitating regulatory engagement, and derisking the path to market. Our experienced teams, advanced instrumentation, and quality-first philosophy ensure that your antibody product is characterized with the scientific depth and regulatory relevance required for successful progression.
Get in touch today to discuss how our Antibody CMC Analytical Support Service can be tailored to accelerate and de-risk your development pipeline—delivering actionable insights at every stage of your biologics journey.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

