Drug Substance & Drug Product Manufacturing Service
Comprehensive Manufacturing Solutions from API to Final Formulation
As the biotechnology sector continues to innovate with complex biologics and next-generation therapeutic antibodies, the need for integrated manufacturing solutions encompassing both drug substance (DS) and drug product (DP) is more crucial than ever. At Creative Biolabs, we provide end-to-end DS and DP manufacturing services designed to support projects from early-phase material supply through late-stage commercial readiness. With a robust infrastructure and deep regulatory insight, we enable seamless transitions between upstream production, downstream purification, and final product fill-finish.
Our Distinctive Manufacturing Platforms – Built for Flexibility and Speed
Creative Biolabs has established purpose-built platforms to accommodate the unique characteristics of biologic drug development. Our modular and scalable manufacturing systems are engineered for flexibility, offering both non-GMP pilot scale runs for feasibility or tox studies and GMP-compliant production suites for commercial manufacturing.
Our core manufacturing platforms include:
- Single-Use Bioreactor Systems (SUBs): Ideal for microbial or mammalian expression, ranging from 2 L to 2,000 L scale.
- Closed-System Processing: Minimizes cross-contamination risk while improving operational efficiency for aseptic production.
- Integrated DS-to-DP Transition Modules: Custom-designed to connect downstream purification directly with formulation and filling units.
- Automated Filling Systems: Suitable for both liquid and lyophilized products in vials, prefilled syringes, or cartridges.
These platforms are fully adaptable to a variety of modalities including monoclonal antibodies (mAbs), fusion proteins, bispecifics, antibody-drug conjugates (ADCs), and other recombinant biologics.
Advanced Technologies Supporting Manufacturing Precision
To ensure batch consistency and product integrity, Creative Biolabs incorporates a range of advanced technologies across DS and DP production stages:
- High-Performance Downstream Purification: Multi-step chromatography (Protein A, ion-exchange, HIC, SEC) and tangential flow filtration (TFF) systems tailored for target purity and yield optimization.
- Inline PAT (Process Analytical Technology): Real-time monitoring of critical parameters during fermentation and purification to enable continuous process control.
- Formulation Engineering: Development and optimization of robust formulations to ensure product stability, bioactivity, and delivery compatibility.
- Lyophilization Development & Cycle Optimization: Freeze-drying protocols for DP stabilization with precise control over freezing, drying, and sealing conditions.
- Sterile Fill-Finish: Aseptic filling performed under strict Grade A/B environments with fill volume accuracy and container closure integrity testing.
Each process step is governed by quality systems compliant with ICH, FDA, EMA, and WHO regulatory frameworks.
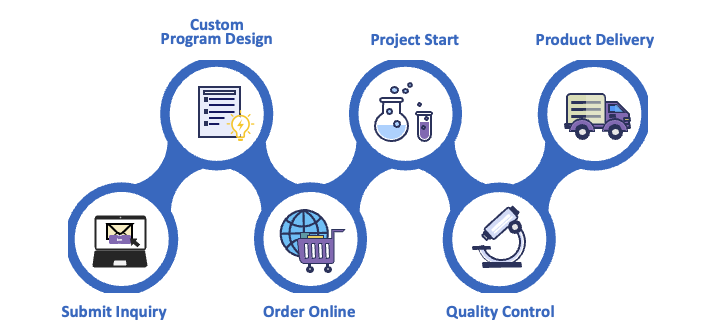
Streamlined Manufacturing Workflow – Phase-Appropriate and Scalable
Our manufacturing workflow is designed to provide clarity and control at every step:
1. Initial Project Evaluation: Comprehensive review of molecular properties, manufacturing needs, and regulatory phase.
2. Tech Transfer & Process Development: Translation of lab-scale protocols to manufacturing scale, including process characterization.
3. Upstream Cell Culture/Fermentation: Cell line expansion, fed-batch or perfusion culture under controlled parameters.
4. Downstream Purification: Multi-step purification yielding DS with desired purity and quality attributes.
5. Formulation and DP Preparation: Buffer exchange, stabilizer addition, and preformulation studies for DP.
6. Fill-Finish and Packaging: Final filling into primary containers, visual inspection, labeling, and secondary packaging.
7. QA/QC and Batch Release: GMP-compliant batch review, testing, and certification of CoAs and batch records.
Frequently Asked Questions
Q1: Can Creative Biolabs manufacture both non-GMP and GMP batches for my antibody product?
A1: Yes, we offer both non-GMP batches for R&D or tox studies, and full GMP-compliant batches for commercial use. Transitions between the two are fully managed.
Q2: What container types can you support for drug product filling?
A2: We support filling into glass vials, prefilled syringes (PFS), cartridges, and lyophilized formats. Container selection can be customized based on product profile and delivery route.
Q3: Do you offer formulation development as part of DP manufacturing?
A3: Absolutely. We provide formulation screening, excipient compatibility studies, and stress testing to ensure DP stability and efficacy.
Q4: Can I transfer an existing DS process to your site?
A4: Yes, we have extensive experience in process transfer and optimization. Our team will work closely with you to ensure seamless tech transfer and scale-up.
Partnering with Creative Biolabs – A Trusted Manufacturing Ally
Choosing Creative Biolabs as your manufacturing partner means entrusting your project to a team with proven expertise, regulatory foresight, and a commitment to quality. Our end-to-end DS and DP manufacturing services are backed by:
GMP-Certified Facilities: Internationally compliant infrastructure for commercial manufacturing.
Dedicated Project Managers: Point-of-contact oversight ensures coordination, transparency, and real-time communication.
Risk-Based Approach: Our manufacturing strategy emphasizes proactive risk identification and mitigation.
Rapid Tech Transfer Capabilities: Minimize project downtime with streamlined onboarding and flexible scale adjustments.
Global Regulatory Acumen: Extensive support for IND, IMPD, and BLA/MAA submissions.
Let Creative Biolabs support your biologics journey from active ingredient synthesis to final drug product. We invite you to discuss how our tailored manufacturing solutions can bring your antibody or protein therapy closer to market with confidence and clarity.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

