Antibody Product focused DS & DP Evaluation Service
Ensuring Biologic Quality from Manufacturing to Final Dosage
In the development of monoclonal antibodies and related biologics, the distinction between drug substance (DS) and drug product (DP) evaluations is crucial. Drug substance refers to the purified, concentrated antibody bulk before final formulation, while drug product includes the formulated antibody in its final container, ready for administration. Each requires meticulous quality assessment to ensure the biologic maintains its stability, efficacy, and safety throughout its lifecycle. Creative Biolabs provides a specialized antibody product-focused DS and DP evaluation service, offering an integrated suite of analytical and functional studies aligned with global regulatory requirements.
Full-Spectrum Assessment Covering DS and DP Stages
Our evaluation platform spans from bulk substance characterization to finished product release testing. Services are modular yet harmonized to track critical quality attributes (CQAs) through the full continuum of product development. Our key offerings include:
- DS Characterization: Includes physicochemical properties, purity, aggregation status, and activity prior to formulation. Analytical techniques are applied to define molecular integrity and consistency across batches.
- DP Stability and Formulation Assessment: Includes forced degradation studies, excipient compatibility, and assessment of packaging-material interactions.
- Comparability Studies: For process changes or manufacturing site transfers, we support bridging studies to ensure consistent product profile at both DS and DP levels.
- Release Testing for Use: Including appearance, pH, osmolality, sterility, particulate matter, and potency testing.
- Container Closure Integrity Testing (CCIT): Ensures DP packaging maintains sterility and prevents contamination during shelf life.
- Cold Chain and Thermal Stress Studies: Evaluate temperature excursion impacts on DP integrity during shipping or long-term storage.
Our assessments can be customized based on antibody isotype, formulation strategy, or specific stability concerns from your process.
Advanced Methodologies for Comprehensive Analysis
Creative Biolabs integrates a portfolio of cutting-edge analytical techniques tailored for biologic DS and DP evaluation, such as:
- Mass Spectrometry (LC-MS/MS): For primary structure confirmation, PTM profiling, and degradation mapping.
- SEC-HPLC and AUC: For aggregation detection and size distribution.
- CD and DSC Analysis: Evaluate secondary structure and thermal stability of DS and DP formulations.
- Bioactivity Assays: Including cell-based assays or binding ELISAs to confirm biological function post-formulation.
- Subvisible Particle Analysis: Per USP <788>, using light obscuration and micro-flow imaging techniques.
- Forced Degradation Panel: Covers thermal, photolytic, oxidative, and pH stress conditions to map degradation pathways.
- Leachables and Extractables Testing: For DP packaging components to ensure no adverse interaction with the antibody.
Robust, Transparent Evaluation Workflow
We follow a stepwise, science-driven approach to DS and DP evaluation that ensures alignment with your regulatory and CMC strategy:
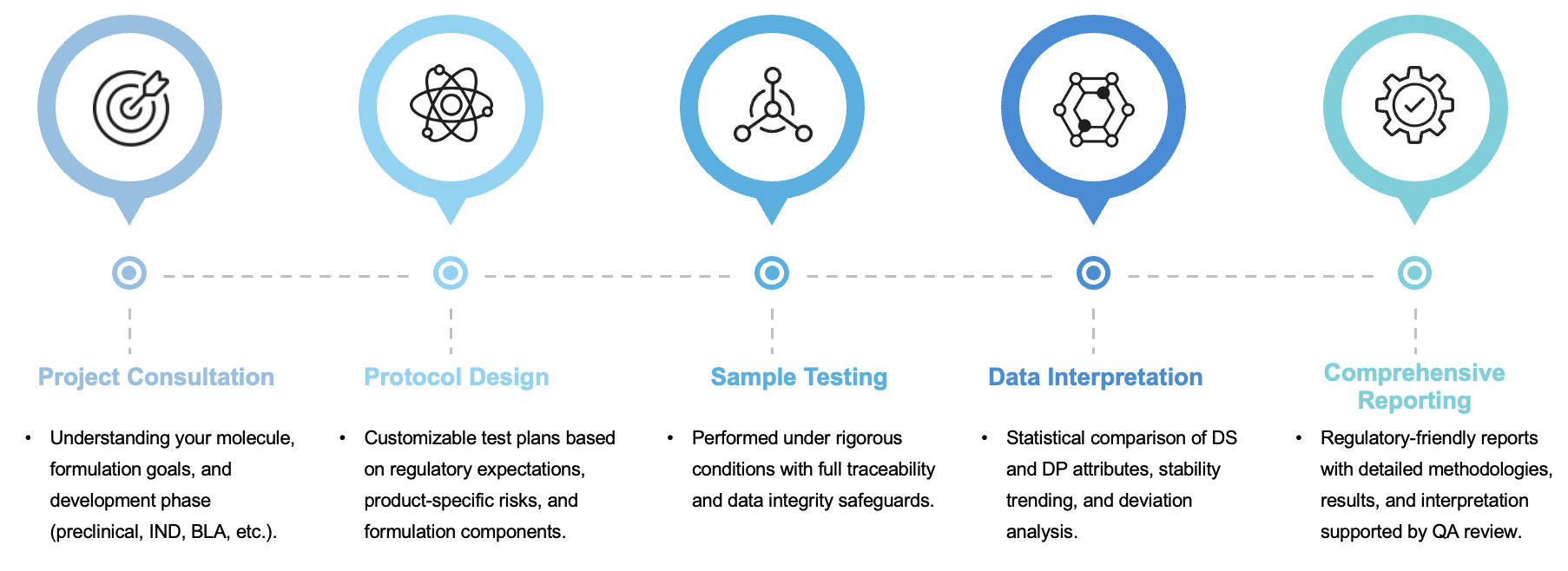
For projects at critical milestones, we offer expedited timelines and phase-appropriate documentation to support submissions.
Key Benefits of Our Evaluation Service
What sets our DS and DP evaluation services apart is the seamless integration of analytical precision, regulatory foresight, and biologic-specific know-how:
Holistic Coverage from Bulk to Vial
Comprehensive testing from upstream substance to final packaged product.
Expertise in Formulated Biologics
Familiarity with antibody-excipient interactions and real-world formulation challenges.
Adaptable to All Antibody Formats
IgGs, bispecifics, ADCs, VHH, and Fc-fusion proteins are supported.
Alignment with Global Guidelines
All methods designed with FDA, EMA, ICH, and WHO expectations in mind.
Scalability Across Development Phases
From early research-grade DS characterization to GMP DP lot release support.
Partner with Creative Biolabs for Product Confidence
Our antibody-focused DS and DP evaluation platform is backed by a multidisciplinary team with deep experience in therapeutic antibody development and CMC analytics. Whether you're validating a formulation for IND filing or preparing for commercial batch release, Creative Biolabs delivers the expertise, infrastructure, and regulatory alignment to support your program at every step.
Contact us today to discuss how our customized Antibody Product-Focused DS and DP Evaluation Service can accelerate your path from formulation to patient-ready drug product — with the analytical rigor your molecule deserves.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

