Plasmid Product Stability Evaluation Service
Service Background — Stability Is the Cornerstone of Plasmid Performance
In the context of plasmid-based therapeutics, including DNA vaccines, gene therapies, and engineered cell platforms, product stability is far more than a shelf-life parameter—it's a decisive factor in regulatory success, clinical efficacy, and logistical planning. Plasmid DNA, although generally regarded as a stable molecule, can degrade or undergo topological changes under suboptimal storage, handling, or formulation conditions. These alterations can impair biological activity, reduce transfection efficiency, or compromise safety.
Creative Biolabs offers a comprehensive Plasmid Product Stability Evaluation Service to rigorously assess the structural and functional robustness of plasmid DNA over time and under stress conditions. This ensures that each product remains consistent, potent, and compliant throughout its lifecycle.
Core Services
Our stability assessment platform simulates both real-time and accelerated conditions that mimic long-term storage, transportation stress, and processing environments. We tailor studies to match your regulatory and operational needs. Key evaluations include:
Accelerated and Long-Term Stability Testing
- Evaluation of plasmid integrity over weeks to months at various storage temperatures (e.g., −80°C, −20°C, 2–8°C, 25°C, 40°C).
Freeze-Thaw Cycle Resistance
- Analysis of structural fidelity and supercoiling ratio after repeated freeze-thaw exposures.
pH and Buffer Compatibility Profiling
- Assessment of plasmid stability across different formulations to guide excipient selection.
Photostability Studies
- Exposure to controlled light conditions per ICH Q1B to determine light-induced degradation risks.
Agitation and Vibration Stress
- Simulations of transportation-related motion to detect shear-induced conformational change.
Residual Activity Assessment Post-Stress
- Functional testing via cell-based transfection or reporter gene expression to verify biological potency post-exposure.
Packaging and Container Closure Impact
- Evaluation of DNA integrity across various container types and sealing materials under stress conditions.
Analytical Technologies: Advanced Tools for Stability Characterization
To ensure precision and reproducibility, we employ a multi-platform analytical strategy integrating orthogonal technologies:
- Differential Scanning Calorimetry (DSC): Assesses thermal stability and denaturation profiles of plasmid backbones.
- NanoDrop and Fluorometric Quantification: Quantifies plasmid content and purity at each time point with high sensitivity.
- Agarose and Capillary Electrophoresis: Detects conformational shifts between supercoiled, open circular, and linear forms.
- Spectroscopic Profiling (UV/Vis, CD): Evaluates structural integrity and base stacking interactions during degradation.
- qPCR-based Integrity Assay: Confirms the stability of functional sequences including promoters, ORFs, and regulatory elements.
- Transfection Efficiency Testing: Benchmarked against fresh control batches to determine loss of activity.
- HPLC/UPLC for Degradant Detection: Identifies emerging low-molecular-weight species or formulation instability markers.
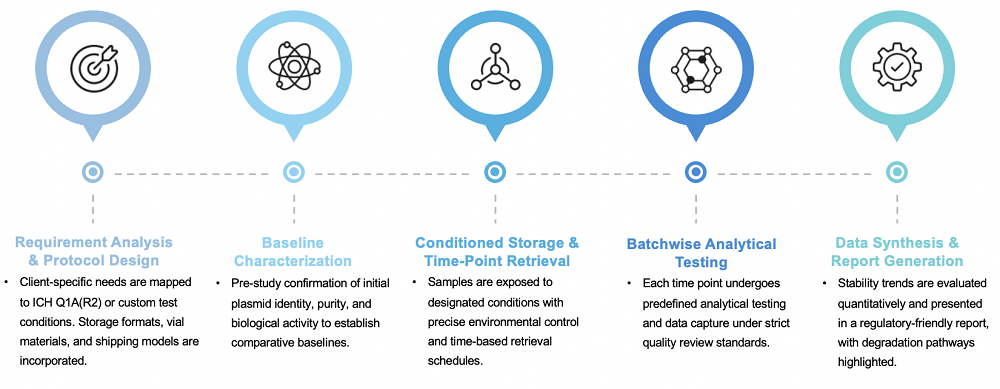
Service Workflow
Our structured evaluation process ensures that each stage of the study is transparent, trackable, and tailored to product requirements:
Why Choose Creative Biolabs
What sets our plasmid product stability evaluation apart is not only our technical infrastructure but our ability to generate decision-enabling insights. Benefits of choosing Creative Biolabs include:
Tailored Study Design
We customize testing conditions and intervals based on formulation type, delivery platform, and therapeutic context.
Cross-Platform Data Integration
We combine structural, physicochemical, and biological datasets for a 360° understanding of product behavior.
ICH and Pharmacopeia-Aligned Protocols
Our methods adhere to global regulatory expectations, facilitating smoother downstream submissions.
Proactive Risk Identification
Early detection of degradation pathways and instability trends to guide formulation and storage decisions.
High-Throughput Capability
Efficient parallel testing of multiple plasmid candidates or formulations.
Partner With Us
In the high-stakes arena of nucleic acid therapeutics, stability is not just a regulatory checkbox—it's a marker of trust in product performance. Creative Biolabs helps you demonstrate that trust, with data-driven assurance.
Whether you're validating a plasmid for clinical trials or optimizing your cold chain for market launch, we offer a customized and scientifically rigorous path forward. Partner with us for a stability evaluation that aligns with both your science and your strategy.
Contact us today to initiate a consultation and design a stability study tailored to your specific plasmid product.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

