 Loading...
Loading...

Anti-HSPE1 Recombinant Antibody Products
 Loading...
Loading...Anti-HSPE1 Products
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, ELISA
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: WB
- Rabbit Anti-HSPE1 Recombinant Antibody (clone JG82-34) (MRO-0384-CN)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC, IP
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, IP
- Rabbit Anti-HSPE1 Recombinant Antibody (VS13-YC559) (VS13-YC559)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, IHC-P, ICC, IF, IP
-
- Derivation: Phage display library
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: FC, IP, WB
- Anti-HSPE1 Immunohistochemistry Kit (VS-0525-XY3346)
-
- Species Reactivity: Human
- Target: HSPE1
- Application: IHC
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Creative Biolabs provides cutting-edge recombinant antibodies targeting HSPE1, empowering researchers to make groundbreaking discoveries in biomedical science. Utilizing state-of-the-art production platforms and stringent validation protocols, we guarantee antibodies that exceed industry benchmarks for specificity and batch-to-batch consistency. Our comprehensive selection of meticulously engineered reagents offers scientists reliable tools for their most demanding investigations. Beyond supplying these premium research solutions, our expert team delivers personalized experimental guidance to optimize study design and implementation. By choosing our services, you gain more than superior reagents – you acquire a dedicated scientific partner committed to advancing your research goals through innovative technologies and collaborative expertise.
HSPE1: A Key Regulator in Mitochondrial Dysfunction and Disease Pathogenesis
HSPE1, also known as HSP10, has emerged as a significant player in various pathological conditions due to its multifaceted roles in cellular homeostasis. This mitochondrial chaperonin forms a critical partnership with HSP60 to facilitate proper protein folding, with its dysfunction implicated in neurodegenerative disorders like Parkinson's disease where misfolded protein accumulation occurs. In cancer biology, HSPE1 demonstrates dual functionality - while normally acting as a tumor suppressor by maintaining protein quality control, certain malignancies exploit its overexpression to support tumor cell survival under stress conditions. The protein's involvement in apoptosis regulation connects it to autoimmune diseases, particularly through its altered expression patterns observed in rheumatoid arthritis patients. Cardiovascular pathologies including ischemia-reperfusion injury show HSPE1 depletion, suggesting its protective role in mitochondrial integrity during oxidative stress. Recent studies reveal extracellular HSPE1's immunomodulatory properties, potentially contributing to inflammatory bowel diseases when signaling pathways are disrupted. Its essential role in mitochondrial protein import machinery also links HSPE1 deficiencies to rare metabolic disorders characterized by organelle dysfunction. The growing understanding of HSPE1's participation in both intracellular and extracellular processes presents novel therapeutic opportunities, though challenges remain in selectively targeting its various functions across different disease contexts while preserving its crucial housekeeping roles.
Alternative Names
EPF; CPN10; GROES; HSP10
Background
This gene encodes a major heat shock protein which functions as a chaperonin. Its structure consists of a heptameric ring which binds to another heat shock protein in order to form a symmetric, functional heterodimer which enhances protein folding in an ATP-dependent manner. This gene and its co-chaperonin, HSPD1, are arranged in a head-to-head orientation on chromosome 2. Naturally occurring read-through transcription occurs between this locus and the neighboring locus MOBKL3.
Cancer-related genes, Plasma proteins
Intracellular
Cell type enhanced (Proximal tubular cells, Basal prostatic cells)
Low immune cell specificity
Cell line enhanced (Hep G2)
Homoheptamer arranged in a ring structure (PubMed:25918392). 2 heptameric Hsp10 rings interact with a Hsp60 tetradecamer in the structure of a back-to-back double heptameric ring to form the symmetrical football complex (PubMed:25918392).
Chaperone
Anti-HSPE1 rAb Products
Our mission is to drive scientific advancement by offering high-quality recombinant anti-HSPE1 antibodies that integrate reliable performance, competitive value, and expert technical support, all strategically designed to assist researchers at every phase of their work and ensure seamless progress toward their objectives.
Table 1. Featured anti-HSPE1 recombinant antibody products at Creative Biolabs.
| Cat. No. | Product Name | Target Species | Host Species | Applications |
| VS3-FY714 | Recombinant Rabbit Anti-HSPE1 Antibody (clone R02-3K6) | Human; Mouse; Rat | Rabbit | WB; IHC-P; IP |
| MRO-0384-CN | Rabbit Anti-HSPE1 Recombinant Antibody (clone JG82-34) | Human; Mouse; Rat | Rabbit | WB; IHC; IP |
| MOB-0723MZ | Recombinant Mouse Anti-Human HSPE1 Antibody (clone M1.4) | Human | Mouse | WB |
Customer Reviews

Recombinant Rabbit Anti-HSPE1 Antibody (clone R02-3K6) (CAT#: VS3-FY714)

Rabbit Anti-HSPE1 Recombinant Antibody (clone JG82-34) (CAT#: MRO-0384-CN)

Recombinant Mouse Anti-Human HSPE1 Antibody (clone M1.4) (CAT#: MOB-0723MZ)
rAb Production
With extensive experience in developing and optimizing recombinant antibodies, we are dedicated to delivering high-quality products efficiently. Our professional service ensures that researchers receive reliable antibody solutions within a short turnaround time.
Featured Anti-HSPE1 Recombinant Antibody Production Platforms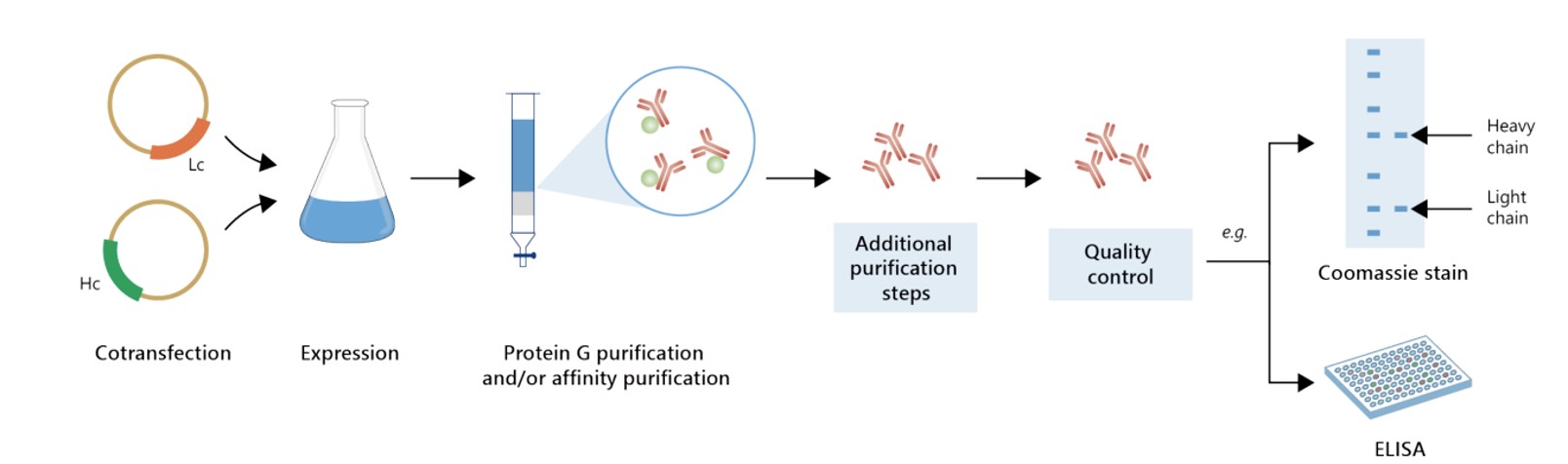
Fig.1 Milligram-scale recombinant antibody production.
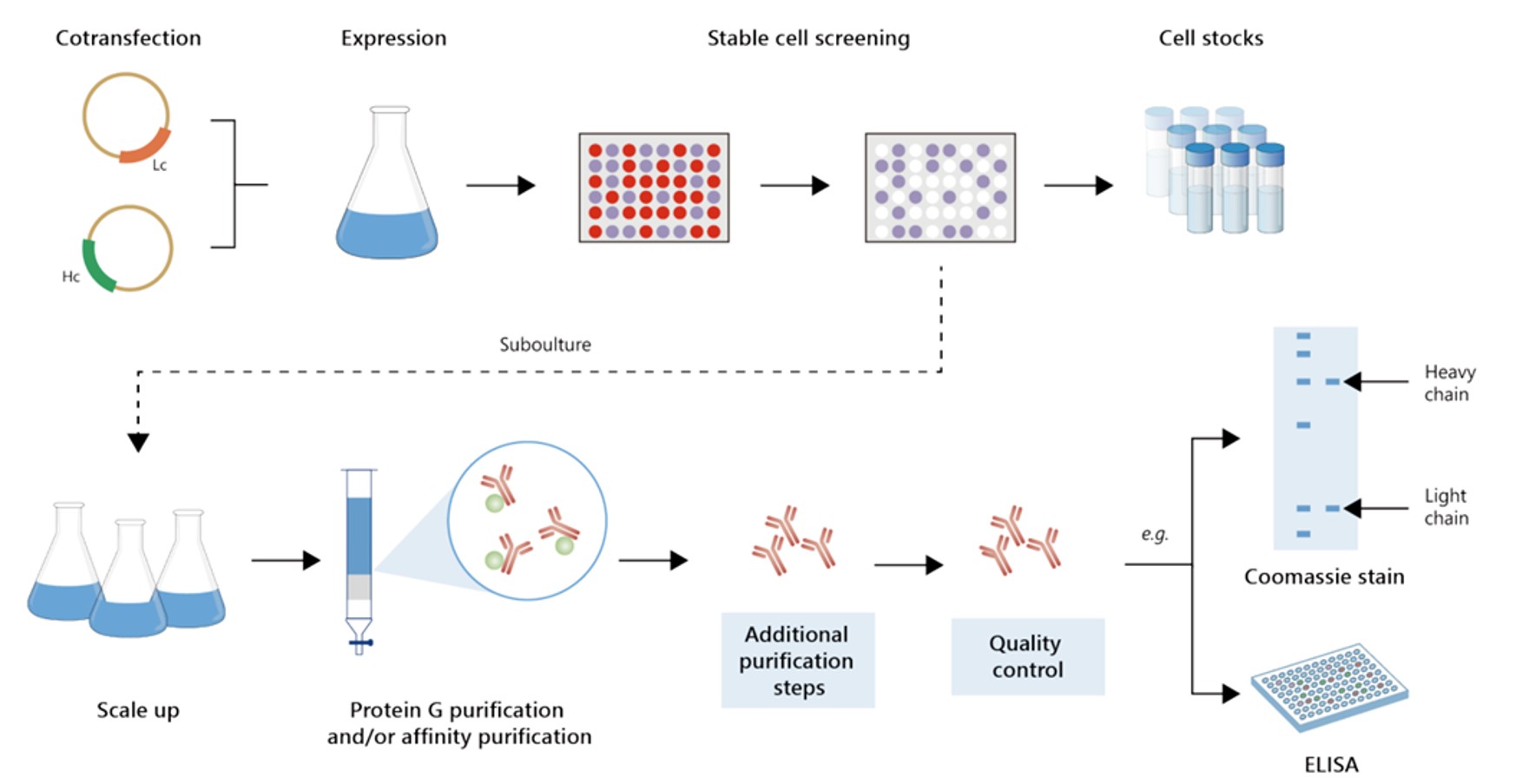 Fig.2 Gram-scale recombinant antibody production.
Fig.2 Gram-scale recombinant antibody production.
rAb Modalities
Creative Biolabs empowers scientists with an unparalleled selection of premium engineered antibodies available in diverse configurations, developed through our mastery of cutting-edge antibody modification technologies. Our specialists excel in creating adaptable research solutions, with every antibody product complemented by fully customizable development services designed to address your project's specific biological challenges and experimental objectives.
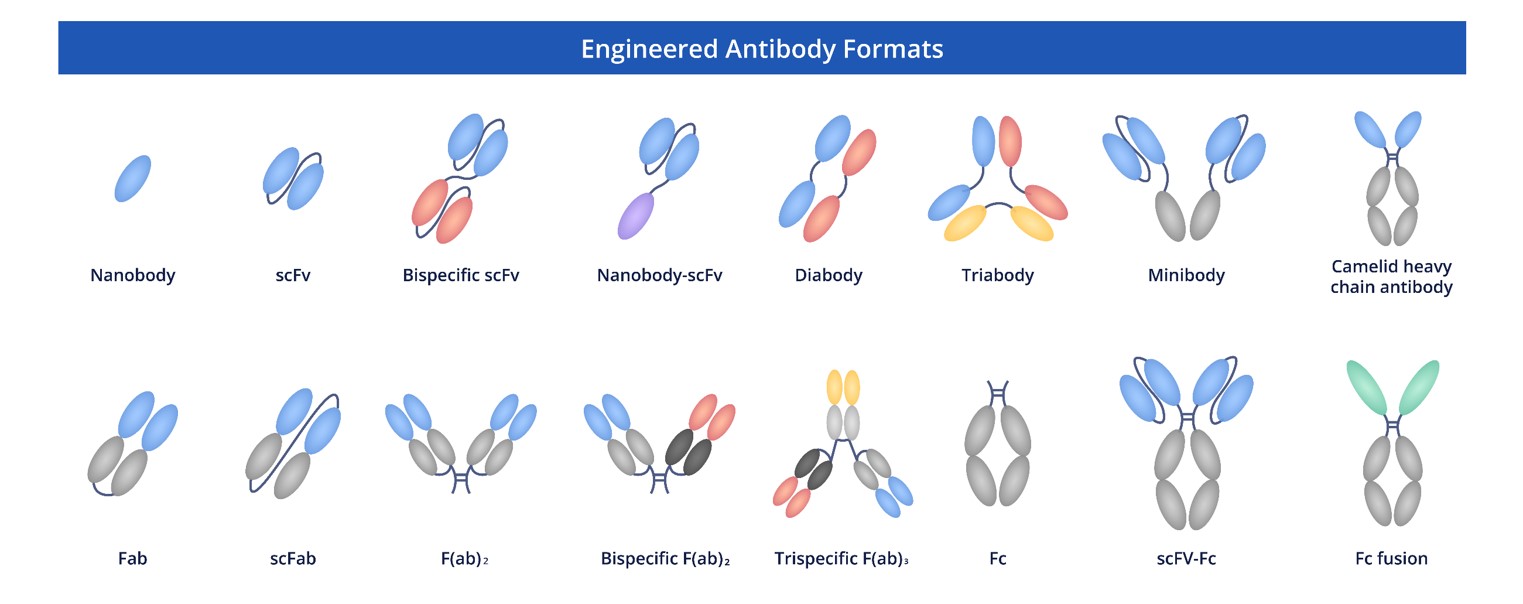 Fig.3 Full Length Anti-HSPE1 Recombinant Antibody Production and Modalities.
Fig.3 Full Length Anti-HSPE1 Recombinant Antibody Production and Modalities.
For comprehensive information regarding the HSPE1 target or technical assistance with your studies, our expert team welcomes your communication via phone or email. We provide tailored guidance to address experimental challenges and optimize research outcomes - please reach out to us for personalized support to advance your scientific investigations efficiently.



