Anti-Human IGF1 and IGF2 Recombinant Antibody (TAB-475CQ)
CAT#: TAB-475CQ
Recombinant monoclonal antibody to IGF1 and IGF2. This antibody is a monoclonal antibody that specifically targets and binds to IGF-1R, blocking its activation by IGF-1 and IGF-2.
Specifications
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Humanized
- Type
- IgG1, λ1
- Specificity
- Human IGF1 and IGF2
- Species Reactivity
- Human
- Applications
- Used for immunoassay techniques such as: Enzyme-Linked Immunosorbent Assay; Immunohistochemistry; Immunofluorescence; Immunoprecipitation; Flow Cytometry; Neutralization
- Conjugate
- Unconjugated
- Related Disease
- Solid tumors
Product Property
- Purity
- > 95% as determined by SDS-PAGE
- Storage
- Store at -20°C for long-term storage. Avoid freeze/thaw cycles.
Target
Customer Review
There are currently no Customer reviews or questions for TAB-475CQ. Click the button above to contact us or submit your feedback about this product.
 Laura Scott
Laura Scott18.Oct,23
 David Young
David Young05.Dec,22
 Megan Martinez
Megan Martinez09.Jul,21
Q&As
-
Is the anti-IGF1 and IGF2 therapeutic antibody suitable for use in Western blotting?
A: Yes, the anti-Xentuzumab therapeutic antibody (TAB-475CQ) is suitable for use in Western blotting. It provides specific binding to Xentuzumab, allowing for reliable detection in Western blot assays.
-
What are the storage recommendations for the anti-IGF1 and IGF2 therapeutic antibody?
A: The recommended storage condition for the anti-Xentuzumab therapeutic antibody (TAB-475CQ) is at -20°C or lower. For short-term storage, it can be kept at 2-8°C. To ensure stability, avoid repeated freeze-thaw cycles.
-
Can the anti-IGF1 and IGF2 therapeutic antibody be used in immunoprecipitation assays?
A: Yes, the anti-Xentuzumab therapeutic antibody (TAB-475CQ) can be used in immunoprecipitation assays. It provides specific binding to Xentuzumab, enabling the successful precipitation of the target protein from complex mixtures.
-
Is the anti-IGF1 and IGF2 therapeutic antibody effective in ELISA applications?
A: Yes, the anti-Xentuzumab therapeutic antibody (TAB-475CQ) is effective in ELISA applications. It has been validated for use in such assays and provides reliable detection of Xentuzumab.
-
What is the optimal dilution for using the anti-IGF1 and IGF2 therapeutic antibody in immunofluorescence?
A: The optimal dilution for using the anti-Xentuzumab therapeutic antibody (TAB-475CQ) in immunofluorescence is typically 1:100 to 1:500. It is advisable to perform a dilution series to determine the best working concentration for your specific experimental conditions.
View the frequently asked questions answered by Creative Biolabs Support.
Citations
-
Hirsch, Theo Z., et al. "Integrated genomic analysis identifies driver genes and cisplatin-resistant progenitor phenotype in pediatric liver cancer." Cancer discovery 11.10 (2021): 2524-2543. https://doi.org/10.1158/2159-8290.CD-20-1809The study conducted an integrated genomic analysis of 126 pediatric liver tumors to uncover driver genes and mechanisms associated with cisplatin resistance. The research identified significant molecular heterogeneity in hepatoblastoma subtypes, revealing plasticity between different molecular subgroups such as "hepatocytic," "liver progenitor," and "mesenchymal." The study found that chemotherapy induces a specific mutational signature in "liver progenitor" cells, leading to heavily mutated relapses and metastases. Additionally, the research validated potential therapeutic targets for cisplatin-resistant progenitor cells through drug screening and mouse xenograft experiments.
Creative Biolabs provided the monoclonal human anti-IGF1/IGF2 therapeutic antibody, Xentuzumab (Cat#: TAB-475CQ), which played a critical role in the study's investigation of new therapeutic strategies for pediatric liver cancer. The antibody was tested at a final concentration of 1 μmol/L, resuspended in PBS at 1 mg/mL, to evaluate its efficacy in inhibiting tumor growth. The provision of Xentuzumab by Creative Biolabs was essential in validating its potential as a targeted treatment, particularly for cisplatin-resistant progenitor cells, thereby contributing significantly to the exploration of alternative therapies for this aggressive form of cancer.
Cite This Product
To accurately reference this product in your publication, please use the following citation information:
(Creative Biolabs Cat# TAB-475CQ, RRID: AB_3111962)
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Biosimilar Overview
Please refer to Xentuzumab Overview to learn more about the mechanism of action, clinical projects, and approved drugs of Xentuzumab.
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Protocol & Troubleshooting
We have outlined the assay protocols, covering reagents, solutions, procedures, and troubleshooting tips for common issues in order to better assist clients in conducting experiments with our products. View the full list of Protocol & Troubleshooting.
See other products for "IGF1 and IGF2"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-475CQ | Afuco™ Anti-IGF1 and IGF2 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-475CQ) | ELISA, IHC, FC, IP, IF, Neut | ADCC enhanced antibody |
Popular Products

Application: FuncS, IF, Neut, ELISA, FC, IP, IHC

Application: WB, ELISA, FuncS

Application: ELISA, SPR, Inhib, FuncS

Application: FC, IB, Block, Inhib, FuncS, ELISA, FACS, IP, IF
-2.png)
Application: WB, ELISA

Application: ELISA, FC, WB, FuncS

Application: ELISA

Application: ELISA, FuncS

Application: ELISA, Inhib, FuncS

Application: ELISA, FC, Inhib

Application: IF, IP, Neut, FuncS, ELISA, FC
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



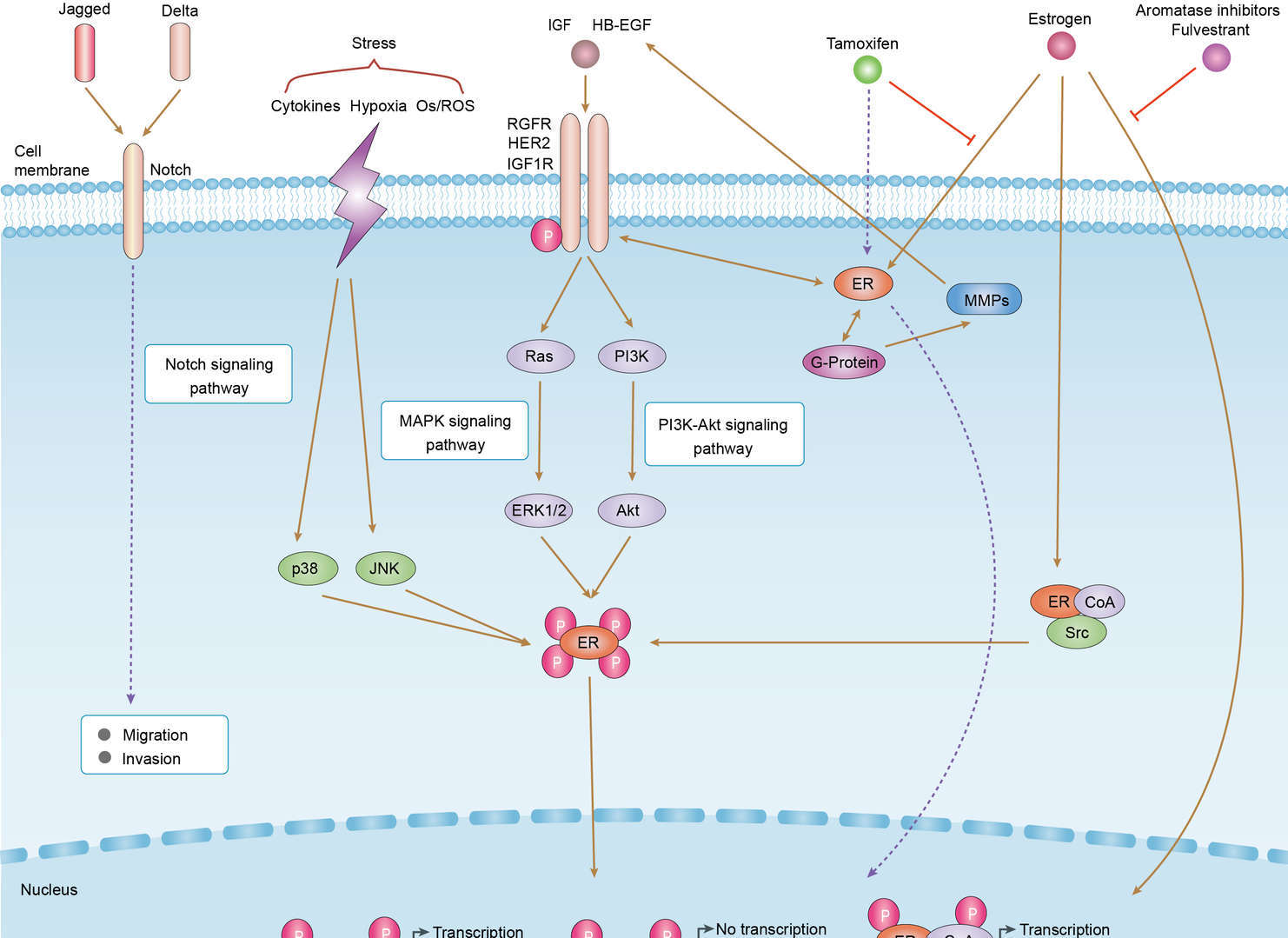 Endocrine Resistance
Endocrine Resistance











