Mouse Anti-IgE Recombinant Antibody (clone 47H4); Fab Fragment
CAT#: HPAB-AP102-YC-F(E)
Provided are anti-IgE antibodies that bind to the M1′ segment of a human IgE. The antibody can be used in treating and preventing IgE-mediated disorders, as well as kits comprising the anti-IgE antibodies.
-1.webp)
-2.webp)
-3.webp)
-4.webp)
-5.webp)
-6.webp)
-7.webp)
-8.webp)
Specifications
- Host Species
- Mouse
- Type
- Mouse Fab
- Specificity
- Human IgE
- Species Reactivity
- Human
- Clone
- 47H4
- Applications
- ELISA
Product Property
- Purity
- >95% as determined by SDS-PAGE and HPLC analysis
- Buffer
- PBS
- Preservative
- No preservatives
- Storage
- Centrifuge briefly prior to opening vial. Store at +4°C short term (1-2 weeks). Aliquot and store at -20°C long term. Avoid repeated freeze/thaw cycles.
Applications
- Application Notes
- ELISA
The peptide ELISA protocol was performed in flat 96-well Streptavidin plates, which were washed 3 × in 0.1% Tween-20/PBS, coated o/n at 4 °C with 30 nM of the biotinylated peptide in PBS, followed by 3 washes. Serum dilutions in 0.1% BSA, 0.1% Tween-20/PBS were added to wells, and incubated while shaking for 1 h at RT, followed by 3 washes with PBS (0.1% Tween-20). For detection, goat anti-mouse IgG + IgM HRPO 1:1000 in 0.1% BSA/0.1% Tween-20/PBS was added for 1 h at RT while shaking, washed 3 times. ABTS with 0.03% H₂O₂was added after 3 washes for visualization followed by incubation at RT (while shaking). The enzymatic reaction was stopped at 30 min with 1% SDS. The optical density was measured at 405 nm with a microwell plate reader Sunrise (Tecan, Switzerland). Prism was used to calculate the EC50 referring to the dilution of the sera, designated peptide titer (or concentrations when using mABs). EC50 values were calculated using non-linear regression analysis with four parameter curve fitting. Total IgE was determined by ELISA MAXTM Standard Set mouse IgE, while ovalbumin-specific IgE was determined by anti-Ovalbumin IgE Elisa kit following the manufacturer's instructions.
Target
- Alternative Names
- Immunoglobulin E
Customer Review
There are currently no Customer reviews or questions for HPAB-AP102-YC-F(E). Click the button above to contact us or submit your feedback about this product.
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
See other products for "Clone 47H4"
- CAT
- Product Name
See other products for "IgE"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-034ZJ | Anti-Human IgE Recombinant Antibody (CMAB007) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-049ZJ | Anti-Human IgE Recombinant Antibody (IGE009) | ELISA, Inhib, FC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-050ZJ | Anti-Human IgE Recombinant Antibody (IGE026) | ELISA, Inhib, FC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-034ZJ-S(P) | Anti-Human IgE Recombinant Antibody scFv Fragment (CMAB007) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-049ZJ-S(P) | Anti-Human IgE Recombinant Antibody scFv Fragment (IGE009) | ELISA, Inhib, FC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-095ZJ | Anti-Human IgE Single Domain Antibody (TAB-095ZJ), Research Grade Biosimilar | ELISA, Inhib, FC | Single domain antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-009LC | Recombinant Anti-Human IgE Antibody (Fab glycosylation/Fc glycosylation) | ELISA, IP | Human antibody |
| Gly-009LC-1 | Recombinant Anti-Human IgE Antibody (Fab glycosylation/Fc glycosylation) | ELISA, IP | Human antibody |
| Gly-010LC-1 | Recombinant Anti-Human IgE Antibody (Fab glycosylation/Fc glycosylation) | ELISA, IP | Human antibody |
| Gly-011LC-1 | Recombinant Anti-Human IgE Antibody (Fab glycosylation/Fc glycosylation) | ELISA, IP | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-156LC | Recombinant Anti-Human IgE Antibody (Fc glycosylation/Non fucosylated) | ELISA | Humanized antibody |
| Gly-156LC-1 | Recombinant Anti-Human IgE Antibody (Fc glycosylation/Non fucosylated) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0126-FY | Human Anti-IgE Recombinant Antibody (HPAB-0126-FY) | FC, ELISA | Humanized IgE |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0126-FY-S(P) | Human Anti-IgE Recombinant Antibody scFv Fragment (HPAB-0126-FY-S(P)) | FC | Humanized scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0126-FY-F(E) | Human Anti-IgE Recombinant Antibody Fab Fragment (HPAB-0126-FY-F(E)) | FC | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0208CQ | Mouse Anti-IgE Recombinant Antibody (clone TES-C21) | ELISA, Block, FuncS | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0209CQ | Human Anti-IgE Recombinant Antibody (clone huTES-C21) | ELISA, Block | Humanized IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0208CQ-F(E) | Mouse Anti-IgE Recombinant Antibody (clone TES-C21); Fab Fragment | ELISA, Block | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0209CQ-F(E) | Human Anti-IgE Recombinant Antibody (clone huTES-C21); Fab Fragment | ELISA, Block | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0209CQ-S(P) | Human Anti-IgE Recombinant Antibody (clone huTES-C21); scFv Fragment | ELISA, Block | Humanized scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0043-WJ | Mouse Anti-IgE Recombinant Antibody (HPAB-0043-WJ) | ELISA, WB, FC, ADCC, FuncS | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0043-WJ-S(P) | Mouse Anti-IgE Recombinant Antibody; scFv Fragment (HPAB-0043-WJ-S(P)) | ELISA, WB | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0043-WJ-F(E) | Mouse Anti-IgE Recombinant Antibody; Fab Fragment (HPAB-0043-WJ-F(E)) | ELISA, WB | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP102-YC | Mouse Anti-IgE Recombinant Antibody (clone 47H4) | ELISA | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP102-YC-S(P) | Mouse Anti-IgE Recombinant Antibody (clone 47H4); scFv Fragment | ELISA | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP103-YC-S(P) | Mouse Anti-IgE Recombinant Antibody (clone 7A6); scFv Fragment | ELISA | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-AP103-YC-F(E) | Mouse Anti-IgE Recombinant Antibody (clone 7A6); Fab Fragment | ELISA | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC10 | SmartAb™ Recombinant Anti-IgE pH-dependent Antibody (VS-0825-YC10) | WB, ELISA, IP, FC, Neut, IF | Human IgG1 kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC165 | SmartAb™ Recombinant Anti-IgE pH-dependent Antibody (Clone Omaizumab) | Inhib, FC, IHC, ELISA | Human IgG1 |
Popular Products

Application: Neut, ELISA, IF, IP, FuncS, FC, ICC

Application: FC, IP, ELISA, Neut, FuncS, IF, ICC

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: WB, IP, IF, FuncS, FC, Neut, ELISA

Application: WB, ELISA, FC, IP, FuncS, IF, Neut

Application: ELISA, IHC

Application: IF, IP, Neut, FuncS, ELISA, FC, WB

Application: FC, IP, ELISA, Neut, FuncS, IF, WB

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: IF, IP, Neut, FuncS, ELISA, FC, ICC

Application: FuncS, Inhib, IP, ELISA
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


-1.png)
-2.png)
-3.png)
-4.png)
-5.png)
-6.png)
-7.png)
-8.png)

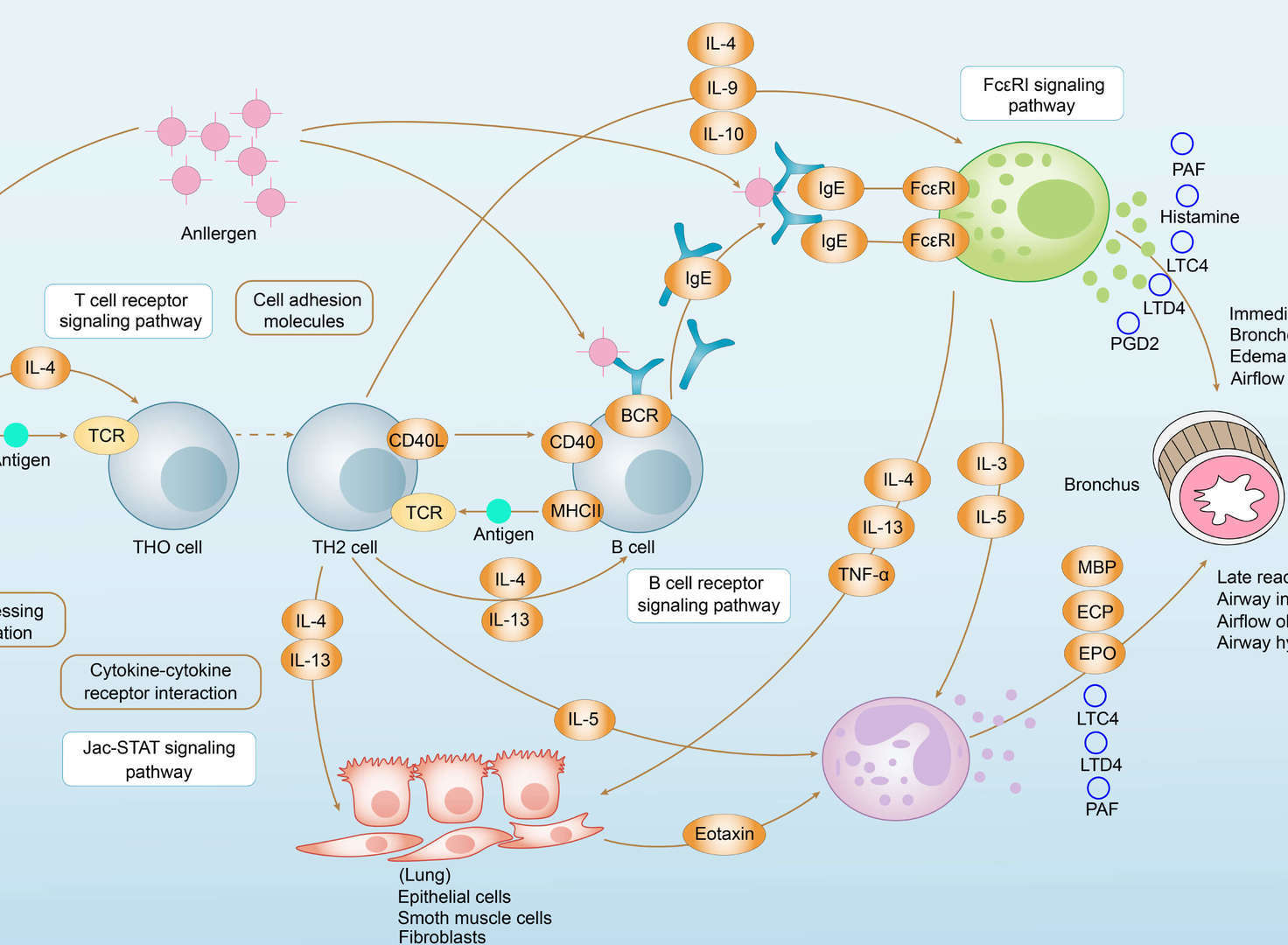 Asthma
Asthma










