Anti-CTLA4 Recombinant Antibody Products
 Loading...
Loading...Anti-CTLA4 Products
 Loading...
Loading...-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG1 - kappa
- Application: WB, FuncS, IF, Neut, ELISA, FC, IP
-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG2
- Application: WB, IF, IP, Neut, FuncS, ELISA, FC
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
-
- Type: Humanized antibody
- Application: ELISA, FC
-
- Type: Humanized antibody
- Application: ELISA, FC
-
- Type: Chimeric antibody (mouse/human)
- Application: ELISA, FC
-
- Type: Chimeric antibody (mouse/human)
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody scFv Fragment (TGN2122.H) (TAB-083MZ-S(P))
-
- Type: Humanized antibody
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody scFv Fragment (TGN2422.H) (TAB-084MZ-S(P))
-
- Type: Humanized antibody
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody scFv Fragment (TGN2122.C) (TAB-085MZ-S(P))
-
- Type: Chimeric antibody (mouse/human)
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody scFv Fragment (TGN2422.C) (TAB-086MZ-S(P))
-
- Type: Chimeric antibody (mouse/human)
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody Fab Fragment (TGN2122.H) (TAB-083MZ-F(E))
-
- Type: Humanized antibody
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody Fab Fragment (TGN2422.H) (TAB-084MZ-F(E))
-
- Type: Humanized antibody
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody Fab Fragment (TGN2122.C) (TAB-085MZ-F(E))
-
- Type: Chimeric antibody (mouse/human)
- Application: ELISA, FC
- Anti-Human CTLA4 Recombinant Antibody Fab Fragment (TGN2422.C) (TAB-086MZ-F(E))
-
- Type: Chimeric antibody (mouse/human)
- Application: ELISA, FC
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human IgG1
- Application: ELISA
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human IgG1
- Application: ELISA
- Anti-Human CTLA4 Recombinant Antibody (Hu-26B) (TAB-094MZ)
-
- Type: Humanized antibody
- Application: ELISA
- Human Anti-CTLA4 Recombinant Antibody; Fab Fragment (TAB-091MZ-F(E)) (TAB-091MZ-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA
- Human Anti-CTLA4 Recombinant Antibody; Fab Fragment (TAB-092MZ-F(E)) (TAB-092MZ-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA
- Anti-Human CTLA4 Recombinant Antibody Fab Fragment (Hu-26B) (TAB-094MZ-F(E))
-
- Type: Humanized antibody
- Application: ELISA
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA
- Anti-Human CTLA4 Recombinant Antibody scFv Fragment (Hu-26B) (TAB-094MZ-S(P))
-
- Type: Humanized antibody
- Application: ELISA
- Human Anti-CTLA4 Recombinant Antibody; Fab Fragment (TAB-088MZ-F(E)) (TAB-088MZ-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA
- Mouse Anti-CTLA4 Recombinant Antibody; Fab Fragment (TAB-089MZ-F(E)) (TAB-089MZ-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CTLA4 Recombinant Antibody; Fab Fragment (TAB-090MZ-F(E)) (TAB-090MZ-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized VHH
- Application: ELISA, Block
- Anti-Human CTLA4 Recombinant Antibody scFv Fragment (Mu-26B) (TAB-093MZ-S(P))
-
- Application: ELISA
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Anti-Human CTLA4 Recombinant Antibody Fab Fragment (Mu-26B) (TAB-093MZ-F(E))
-
- Application: ELISA
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
-
- Species Reactivity: Human
- Type: Camel VHH
- Application: ELISA, Block
- Anti-Human CTLA4 Recombinant Antibody (Mu-26B) (TAB-093MZ)
-
- Application: ELISA
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA
- Mouse Anti-CTLA4 Recombinant Antibody; Fab Fragment (TAB-087MZ-F(E)) (TAB-087MZ-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Recombinant Mouse Anti-CTLA4 Antibody (MOB-0317MC)
-
- Species Reactivity: Human, Rat
- Application: ELISA, FACS
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
-
- Derivation: Phage display library
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Creative Biolabs provides a broad selection of high-quality recombinant antibodies targeting CTLA4, tailored to meet the needs of biomedical researchers and institutions. These antibodies are crafted for maximum specificity and reliability across various applications. Our commitment to advancing scientific research is reflected in the innovative solutions we offer, enabling researchers to drive progress in immunology and cancer studies. By utilizing our CTLA4 antibodies, you can elevate your research and contribute to deeper insights into immune regulation.
CTLA4: A Key Immune Checkpoint of Immune-Related Disease
CTLA4 (cytotoxic T-lymphocyte-associated protein 4) is a crucial immune checkpoint protein involved in regulating immune responses. It is a transmembrane protein consisting of an extracellular domain, a transmembrane domain, and an intracellular domain. The extracellular domain binds to ligands, while the intracellular domain interacts with signaling molecules to modulate T cell activation. Research on CTLA4 is pivotal for understanding immune regulation and tolerance. Blocking CTLA4 has proven effective in cancer immunotherapy by preventing its inhibitory effect and boosting the anti-tumor immune response. Additionally, a deeper understanding of CTLA4's structure and function can aid in developing innovative therapies for a range of immune-related diseases.
Alternative Names
Cytotoxic T-lymphocyte associated protein 4; CD; GSE; GRD4; ALPS5; CD152; CTLA-4; IDDM12; CELIAC3
Background
This gene is a member of the immunoglobulin superfamily and encodes a protein that transmits an inhibitory signal to T cells. The protein contains a V domain, a transmembrane domain, and a cytoplasmic tail. Alternate transcriptional splice variants, encoding different isoforms, have been characterized. The membrane-bound isoform functions as a homodimer interconnected by a disulfide bond, while the soluble isoform functions as a monomer. Mutations in this gene have been associated with insulin-dependent diabetes mellitus, Graves disease, Hashimoto thyroiditis, celiac disease, systemic lupus erythematosus, thyroid-associated orbitopathy, and other autoimmune diseases.
Anti-CTLA4 rAb Products
Our company offers high-quality anti-CTLA4 recombinant antibodies, supporting research and the development of therapies targeting this important molecule.
| Cat. No. | Product Name | Target Species | Host Species | Applications |
| TAB-067 | Anti-Human CTLA4 Recombinant Antibody (Ipilimumab) | Human | Human | WB; FuncS; IF; Neut; ELISA; FC; IP |
| TAB-195CL | Mouse Anti-CTLA4 Recombinant Antibody | Human | Mouse | ELISA |
| TAB-206 | Anti-Human CTLA4 Recombinant Antibody (Ticilimumab (Tremelimumab)) | Human | Human | WB; IF; IP; Neut; FuncS; ELISA; FC |
| HPAB-N0054-YC | Human Anti-CTLA4 Recombinant Antibody (clone 4H9-G5-D5-B1) | Human | Chimeric (mouse/human) | ELISA; FC |
| HPAB-1098-FY | Human Anti-CTLA4 Recombinant Antibody | Human | Human | ELISA |
Creative Quality Control
To meet our customers' expectations for antibody quality, we have implemented a rigorous quality management system. This approach guarantees the excellence of our products, allowing researchers to conduct their work with greater efficiency and ease.
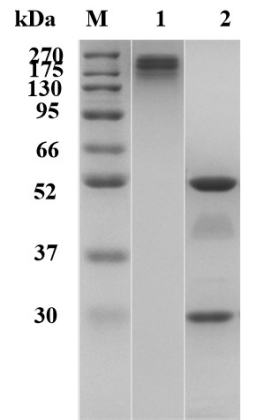 Fig.1 SDS-PAGE analysis of anti-CTLA4 antibody
Fig.1 SDS-PAGE analysis of anti-CTLA4 antibody
(Cat# TAB-206, Creative Biolabs).
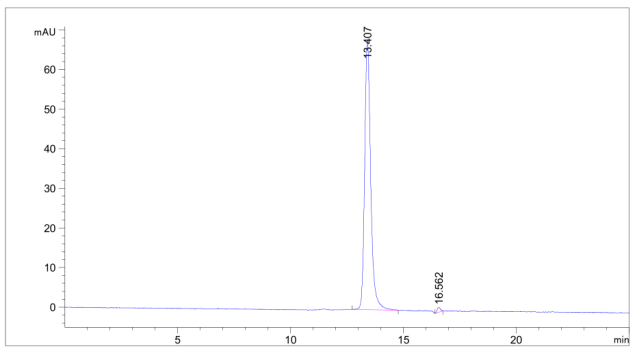 Fig.2 SEC-HPLC analysis of anti-CTLA4 antibody
Fig.2 SEC-HPLC analysis of anti-CTLA4 antibody
(Cat# TAB-206, Creative Biolabs).
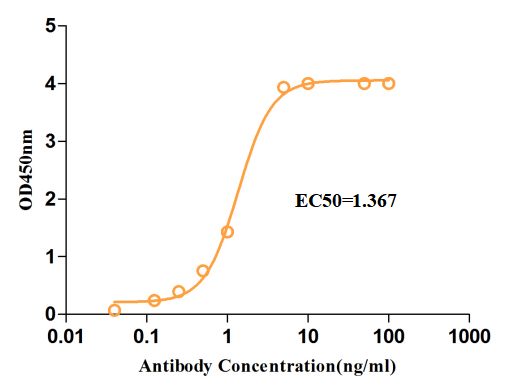 Fig.3 ELISA analysis of anti-CTLA4 antibody
Fig.3 ELISA analysis of anti-CTLA4 antibody
(Cat# TAB-067, Creative Biolabs).
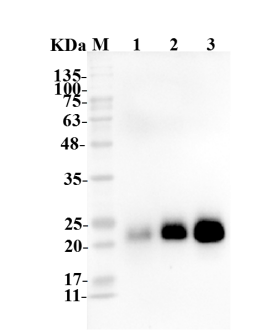 Fig.4 WB analysis of anti-CTLA4 antibody
Fig.4 WB analysis of anti-CTLA4 antibody
(Cat# TAB-067, Creative Biolabs).
Customer Reviews

Anti-Human CTLA4 Recombinant Antibody (Ipilimumab) (Cat#: TAB-067)

Anti-Human CTLA4 Recombinant Antibody (Ticilimumab (Tremelimumab)) (Cat#: TAB-206)

Mouse Anti-CTLA4 Recombinant Antibody (Cat#: TAB-195CL)
rAb Production
With years of expertise in process optimization, including the development of expression vectors, screening of transfected cell lines, and antibody purification, we are committed to offering comprehensive services to biomedical researchers. From gene synthesis to the final production of high-quality antibodies, we strive to support your research with excellence at every step.
Featured Anti-CTLA4 Recombinant Antibody Production Platforms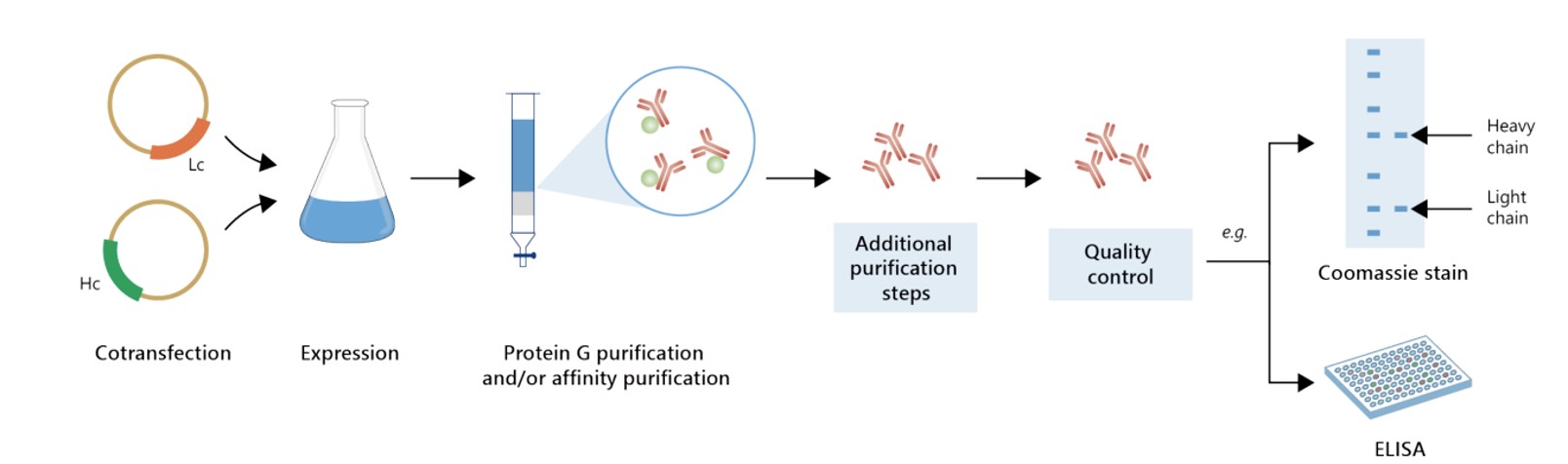
Fig.5 Milligram-scale recombinant antibody production.
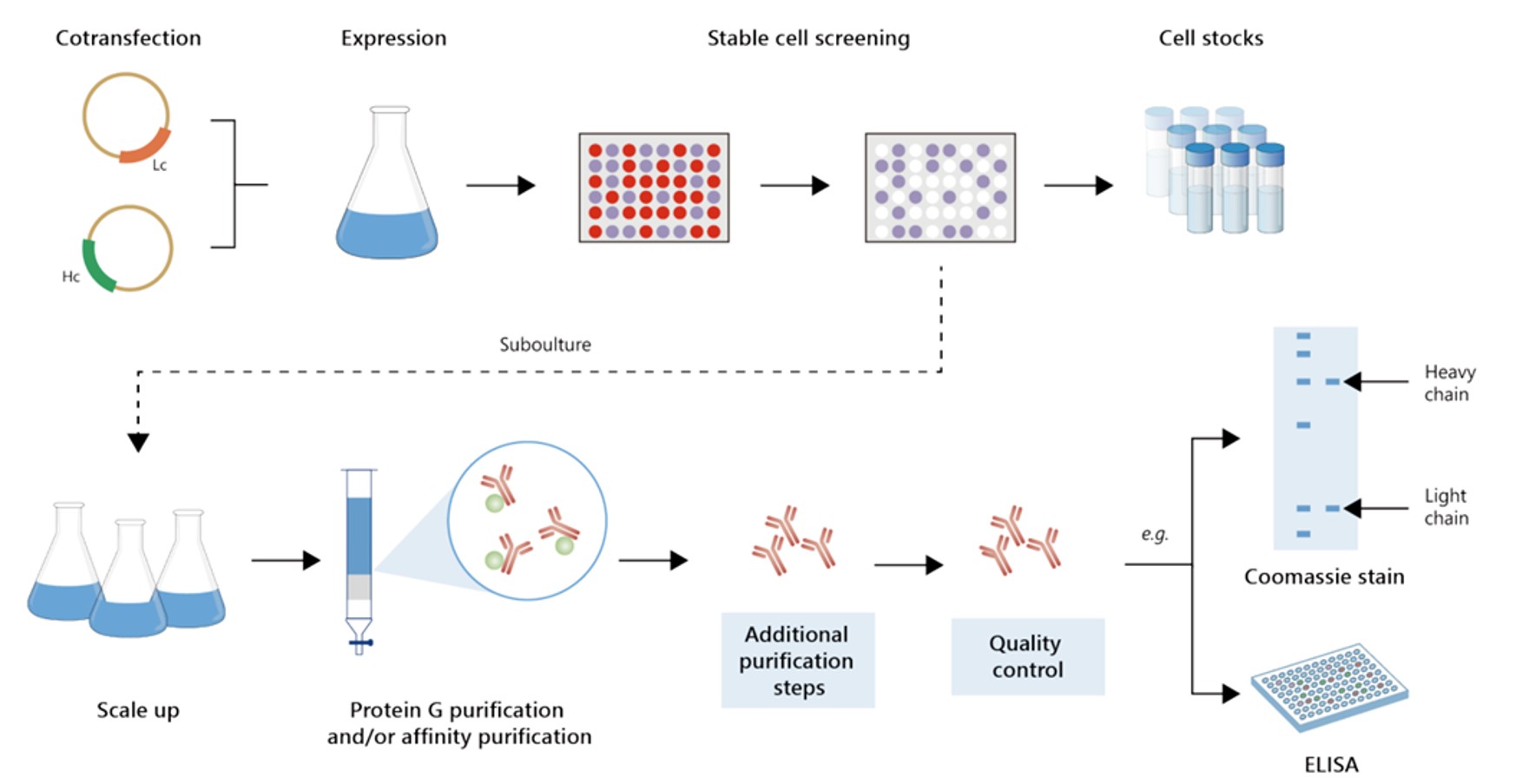 Fig.6 Gram-scale recombinant antibody production
Fig.6 Gram-scale recombinant antibody production
rAb Modalities
We are fully dedicated to delivering premium recombinant antibodies in multiple formats to researchers. Our goal is to address the varied needs of our clients and equip them with the crucial tools required to advance their research projects.
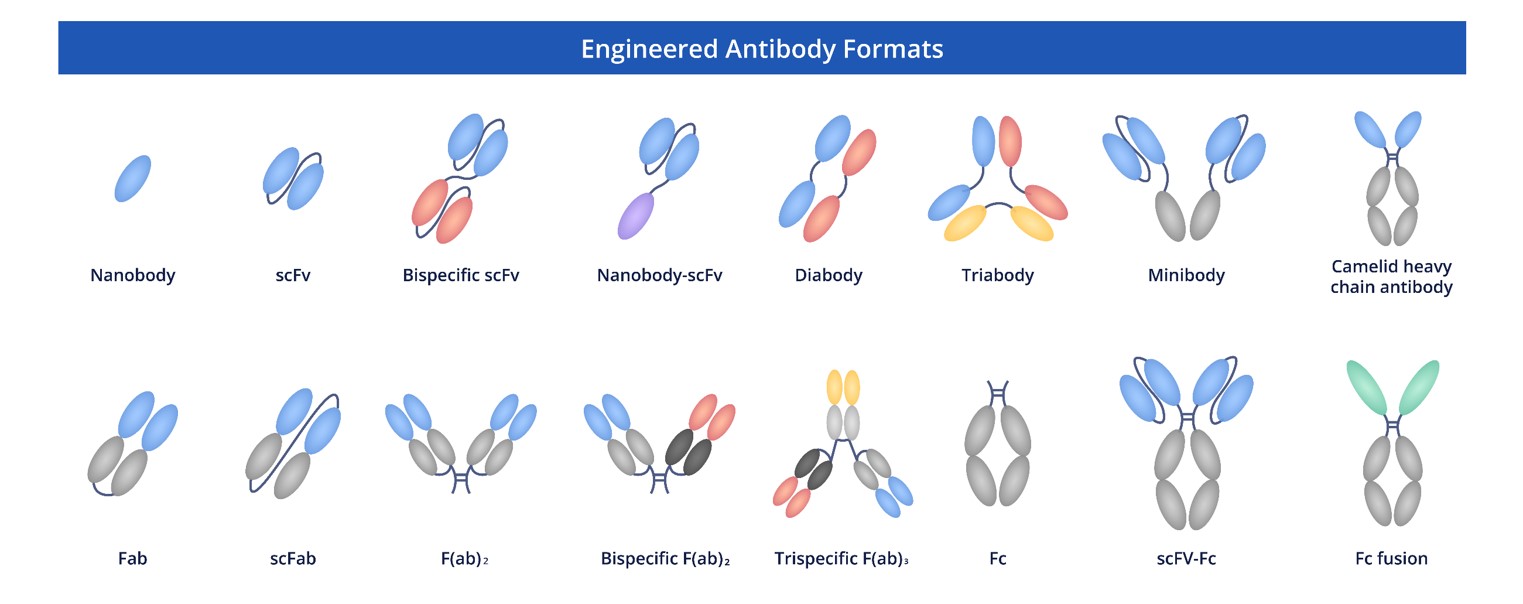 Fig.7 Full Length Anti-CTLA4 Recombinant Antibody Production and Modalities
Fig.7 Full Length Anti-CTLA4 Recombinant Antibody Production and Modalities
Drug Information Targeting CTLA4
Table 1. Therapeutic approaches targeting CTLA4 in clinical development.
| Research phase | Company | Classification | Indications | Details |
| Launched - 2022 | Akeso Biopharma | Biologics | Cancer | This is a bispecific antibody against PD-1 and CTLA-4 developed by Akeso Biopharma, first-time approved in China in 2022 for the treatment of relapsed/metastatic cervical cancer. |
| Launched - 2022 | Amgen | Biologics | Cancer | It is an anti-CTLA-4 antibody developed at AstraZeneca first approved and launched in 2022 in the U.S. for the treatment of adult patients with unresectable hepatocellular carcinoma (HCC) followed by durvalumab. |
| Launched - 2011 | Bristol-Myers Squibb | Biologics |
Acute myeloid leukemia; Cancer |
It is a fully human antibody targeting the CTLA-4 receptor, is available in the U.S. and the E.U. for the treatment of unresectable or metastatic melanoma in patients 12 years and older. |
| Launched - 2006 | Bristol-Myers Squibb | Biologics | Alopecia | This is the first selective modulator of a co-stimulatory signal required for full T-cell activation, was launched in the U.S. by Bristol-Myers Squibb in an intravenous formulation for the treatment of rheumatoid arthritis (RA). E.U. approval and launch took place in 2007. |
| Phase III | Biocad | Combinations | Melanoma | It is a fixed-dose combination (FDC) of the anti-CTLA-4 monoclonal antibody nurulimab and the anti-PD-1 monoclonal antibody prolgolimab being developed by Biocad as an intravenous immunotherapeutic treatment for melanoma. |
| Phase III | Innovent Biologics | Biologics | Acral lentiginous melanoma | It is a non-innovator form of the anti-CTLA4 antibody Yervoy[R] (ipilimumab), is in phase III clinical development. |
| Phase III | Alphamab (Jiangsu) Biopharmaceuticals | Biologics | Cancer | This is a bispecific antibody targeting PD-L1 and CTLA-4 in phase III clinical trials at Jiangsu Alphamab Biopharmaceuticals for the treatment of advanced squamous non-small cell lung cancer (NSCLC). |
| Phase III | AstraZeneca | Biologics | Cancer | It is a bispecific humanized monoclonal IgG1 antibody targeting programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) developed by MedImmune and its parent company AstraZeneca. |
| Phase III | BioNTech | Biologics | Cancer | It is a humanized monoclonal antibody targeting CTLA-4 in phase III clinical development at OncoC4 and BioNTech for the treatment of patients with metastatic non-small cell lung cancer (NSCLC) whose disease progressed on anti-PD-1/PD-L1 antibody-based therapy. |
| Phase II/III | Agenus | Biologics | Cancer | It is an Fc-engineered human monoclonal antibody against CTLA-4 being developed by Agenus as an intravenous immunotherapeutic for oncological indications. |
| Phase II | Biokin Pharmaceutical | Biologics | Cancer | This is a bispecific antibody against PD-1 and CTLA4 in phase II clinical development at Biokin Pharmaceutical as an anticancer immunotherapeutic. |
| Phase II | Akeso Biopharma | Biologics | Cancer | It is a humanized monoclonal antibody targeting CTLA-4 being developed by Merck & Co. as an agent to be used in combination with pembrolizumab for the potential treatment of advanced solid tumors. |
| Phase II | Xencor | Biologics | Cancer | It is a bispecific monoclonal antibody targeting programmed cell death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) in phase II clinical development at Xencor for the treatment of metastatic castration-resistant prostate cancer (mCRPC) and advanced gynecologic and genitourinary malignancies. |
| Phase II | Agenus | Biologics | Cancer | This is a human monoclonal antibody directed against CTLA-4. |
| Phase I/II | Adagene | Biologics | Cancer | It is an anti-CTLA-4 fully human monoclonal antibody in early clinical development at Adagene for the intravenous treatment of advanced solid tumors, including hepatocellular carcinoma (HCC) and metastatic microsatellite-stable (MSS) colorectal cancer (CRC). The candidate is being evaluated in combination with other agents. |
| Phase I | Alpine Immune Sciences | Biologics | Cancer | It is a PD-L1/CTLA-4 antagonist and PD-L1-dependent CD28 T cell costimulatory, is in early clinical development at AIS Operating (formerly, Alpine Immune Sciences) for the treatment of patients with advanced solid tumors or lymphomas that are refractory or resistant to standard therapy, as single agent and in combination with pembrolizumab. |
| Phase I | MacroGenics | Biologics | Cancer | This is a bispecific tetravalent dual-affinity re-targeting (DART) diabody that targets PD-1 and CTLA-4. The candidate is in early clinical development at MacroGenics for the treatment of patients with unresectable, locally advanced or metastatic solid tumors. |
| Phase I | Biocad | Biologics | Melanoma | It is a human monoclonal antibody against CTLA-4 that was developed by Biocad as an immunotherapeutic for the treatment of unresectable/metastatic melanoma. It underwent phase I clinical evaluation as a first-line single-agent treatment for unresectable/metastatic melanoma. |
| Phase I | Xencor | Biologics | Cancer | It is a bispecific antibody that targets immune checkpoint receptors CTLA-4 and LAG-3, is in early clinical development at Xencor for the treatment of solid tumors. |
If you would like more information about our anti-CTLA4 recombinant antibody products, please don't hesitate to reach out to us at any time. Our team is here to provide assistance and address any inquiries you may have, and we eagerly anticipate the opportunity to establish a mutually beneficial partnership with you.


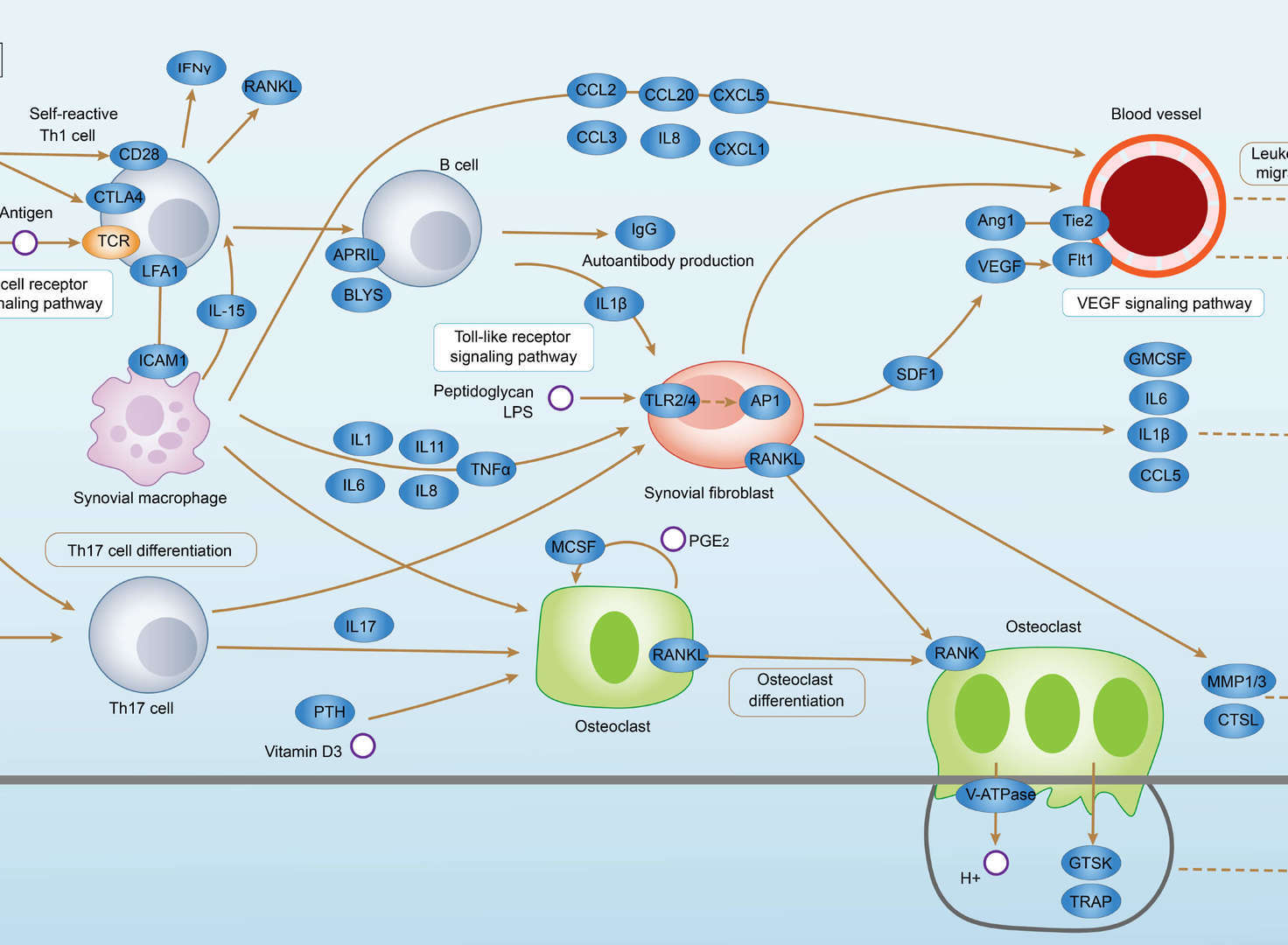 Rheumatoid Arthritis
Rheumatoid Arthritis