 Loading...
Loading...

EIF4E
Anti-EIF4E Recombinant Antibody Products
-
- Derivation: Phage display library
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IP, IHC-P, ICC
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Cancer-related genes, Disease related genes, Human disease related genes, Plasma proteins
Intracellular
Group enriched (Late spermatids, Early spermatids)
Low immune cell specificity
Low cell line specificity
eIF4F is a multi-subunit complex, the composition of which varies with external and internal environmental conditions (PubMed:11408474, PubMed:11879179, PubMed:16271312, PubMed:17631896). It is composed of at least EIF4A, EIF4E and EIF4G1/EIF4G3 (PubMed:8521827, PubMed:11408474, PubMed:11879179, PubMed:12975586). EIF4E is also known to interact with other partners (PubMed:8521827, PubMed:11408474, PubMed:11879179, PubMed:12975586). Interacts with EIF4ENIF1/4E-T; promotes recruitment to P-bodies and import into the nucleus (PubMed:10856257, PubMed:16157702, PubMed:23991149, PubMed:24335285, PubMed:28487484). Hypophosphorylated EIF4EBP1, EIF4EBP2 and EIF4EBP3 compete with EIF4G1/EIF4G3 to interact with EIF4E; insulin stimulated MAP-kinase (MAPK1 and MAPK3) phosphorylation of EIF4EBP1 causes dissociation of the complex allowing EIF4G1/EIF4G3 to bind and consequent initiation of translation (PubMed:8521827, PubMed:16271312, PubMed:21661078, PubMed:24207126, PubMed:25533957, PubMed:25702871). Interacts mutually exclusive with EIF4A1 or EIF4A2 (PubMed:11408474). Interacts with NGDN and PIWIL2 (By similarity). Component of the CYFIP1-EIF4E-FMR1 complex composed of CYFIP, EIF4E and FMR1 (By similarity). Interacts directly with CYFIP1 (By similarity). Interacts with CLOCK (By similarity). Binds to MKNK2 in nucleus (PubMed:12897141). Interacts with LIMD1, WTIP and AJUBA (PubMed:20616046). Interacts with APOBEC3G in an RNA-dependent manner (PubMed:16699599). Interacts with LARP1 (PubMed:20430826). Interacts with METTL3 (PubMed:27117702). Interacts with RBM24; this interaction prevents EIF4E from binding to p53/TP53 mRNA and inhibits the assembly of translation initiation complex (PubMed:29358667). Interacts with DDX3X; interaction is direct and in an RNA-independent manner; this interaction enhances EIF4E cap-binding ability and is required for the repression of cap-dependent translation and the increase of IRES-mediated translation (PubMed:17667941, PubMed:21883093, PubMed:28733330). DDX3X competes with EIF4G1 for interaction with EIF4E (PubMed:17667941, PubMed:21883093). Interacts with BTG4 and CNOT7 (By similarity). (Microbial infection) Interacts with Lassa virus Z protein.
Initiation factor, RNA-binding

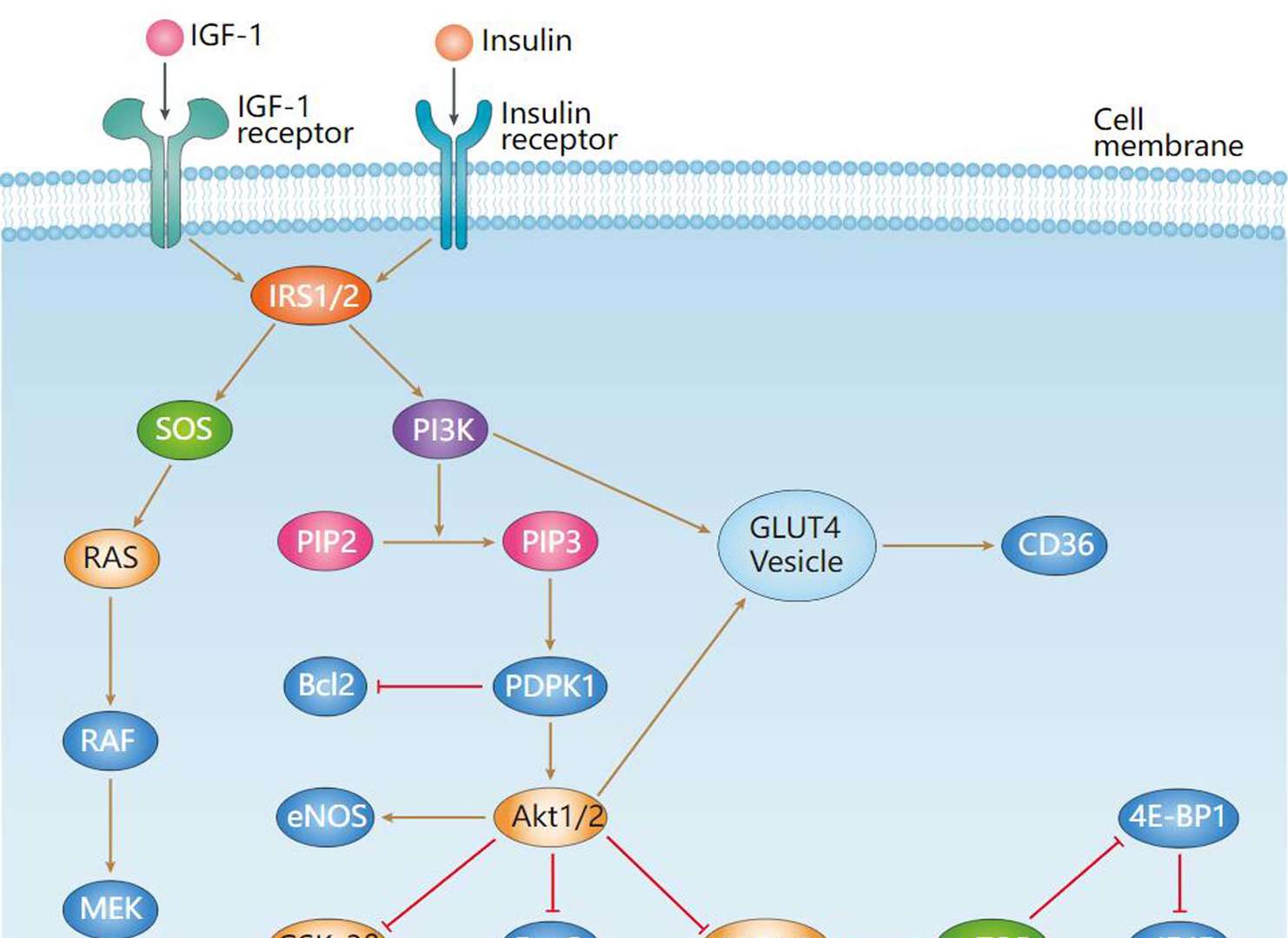 Insulin Signaling Pathway
Insulin Signaling Pathway
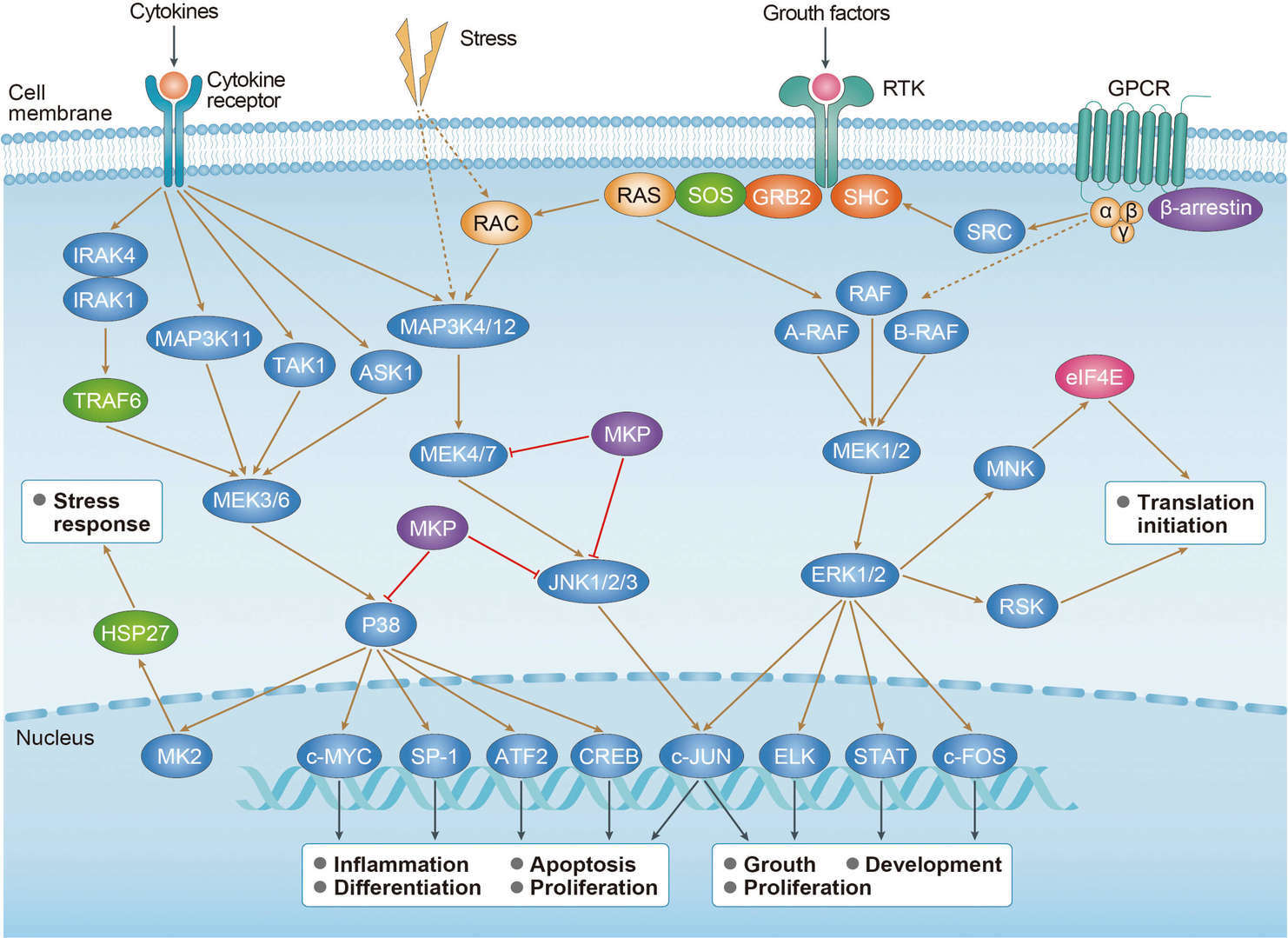 MAPK Signaling Pathway
MAPK Signaling Pathway
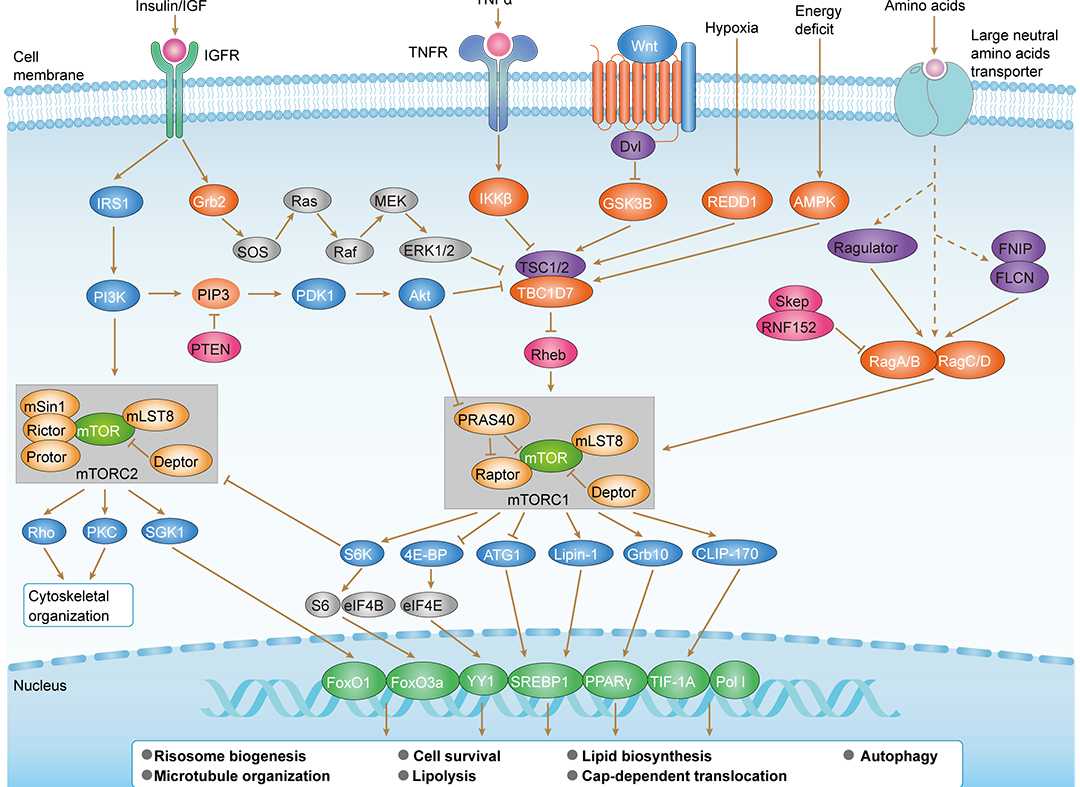 mTOR Signaling Pathway
mTOR Signaling Pathway
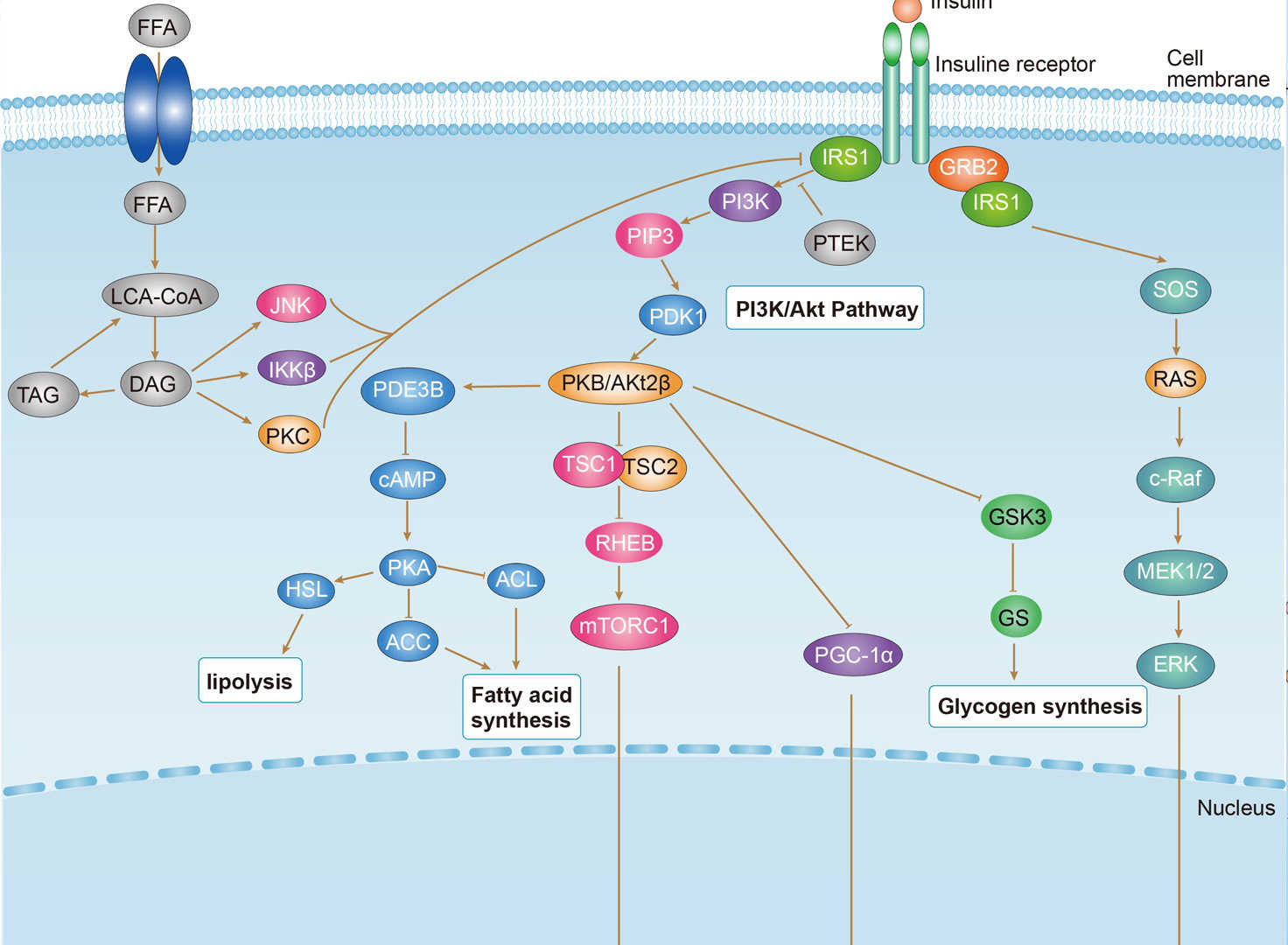 Insulin Resistance
Insulin Resistance