Host Cell Commercial Licensing Service
Introduction
Host cell commercial licensing forms a pivotal part of the biotechnology and pharmaceutical landscape, enabling customized and efficient production of monoclonal antibodies and other biologic modalities through CDMO (Contract Development and Manufacturing Organization) solutions. Creative Biolabs, leveraging its extensive expertise, offers tailored licensing and development solutions to provide high-quality, scalable, and cost-efficient products.
Host Cell Commercial Licensing for Antibody CDMO Solutions
The CHO (Chinese Hamster Ovary) cells stand as the industry gold standard for the production of therapeutic antibodies due to their high yield capabilities and robust post-translational modifications. Creative Biolabs offers a suite of licensed CHO cell lines, which include wild-type and engineered variants. These cell lines are integral to developing antibodies with superior efficacy and stability, optimized for large-scale production.
Key elements of the licensing package include:
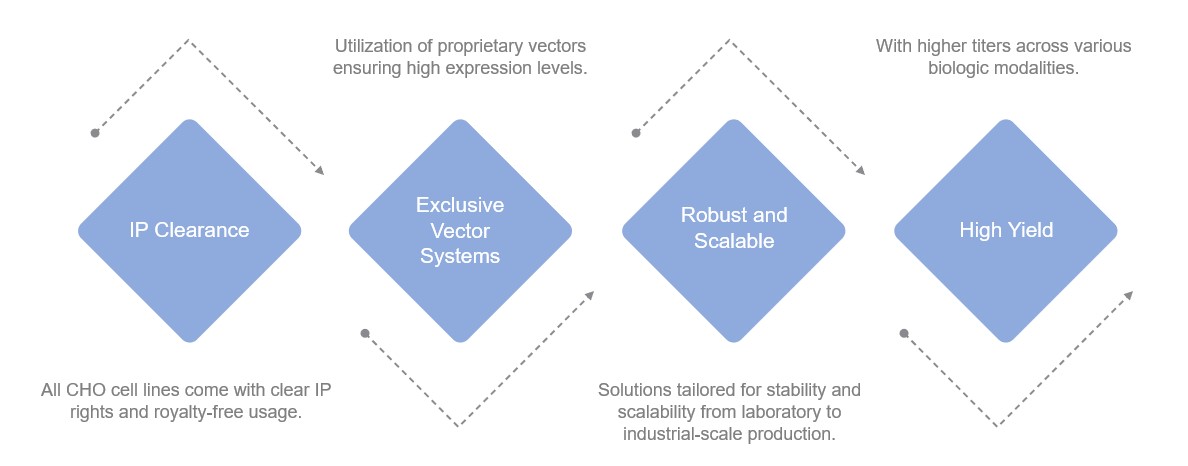
CHO WT: A widely adopted, versatile system for diverse protein expressions.
CHO Engineered Variants: Incorporation of advanced genetic insertions to boost mAb production or enhance other antibody functions.
Deliverables
Creative Biolabs ensures comprehensive deliverables that streamline the transition from development to market. Key deliverables include:
- Licensing of Proprietary CHO Cell Lines.
- Comprehensive Documentation: Including ECACC license agreements, vector synthesis reports, and cGMP cell banking documentation.
- Technical Protocols: Detailed methodologies for cell culture, plasmid construction, and clone screening.
- Technical Support: Continuous support from initial vector design through to scale-up and production, ensuring optimized performance and troubleshooting.
Highlights of Creative Biolabs' Solutions
Applications of Host Cell Commercial Licensing
The applications of host cell commercial licensing are vast, primarily focusing on:
- From monoclonal antibodies to more complex bispecific formats, Creative Biolabs' cell lines support the development of highly effective therapeutic agents.
- Utilizing CHO cell lines for the development and manufacturing of vaccine candidates, ensuring safety and efficacy.
- Production of complex proteins necessary for therapeutic applications, benefiting from the high-fidelity glycosylation patterns of CHO cells.
- The development of producer cell lines for viral vector manufacturing, utilizing advanced genetic modification techniques.
Frequently Asked Questions
Q1: What are the main advantages of using CHO cells for antibody production?
A1: CHO cells provide a stable and high-yield system for producing complex proteins with human-like post-translational modifications, making them ideal for therapeutic antibody production.
Q2: What support is available during the cell line development process?
A2: Creative Biolabs provides full technical support throughout the development process, including vector design, cell culture optimization, and scale-up, ensuring a seamless transition from development to production.
Q3: Are there customizable solutions available?
A3: Yes, Creative Biolabs customizes its licensing and development solutions to meet specific client needs, ensuring the development of cell lines that are tailored to the unique requirements of each project.
Creative Biolabs' host cell commercial licensing solutions offer biopharmaceutical companies a robust platform for the development of therapeutic antibodies and other biologics. With industry-leading cell lines, comprehensive support, and advanced genetic tools, Creative Biolabs stands out as a premier partner in the biotechnology landscape, driving forward the capabilities and success of biopharmaceutical manufacturing.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



