 Loading...
Loading...

TSC1
Anti-TSC1 Recombinant Antibody Products
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG2b
- Application: WB, FC, IHC
-
- Species Reactivity: Human
- Type: Mouse IgG2b
- Application: WB
- Mouse Anti-TSC1 Recombinant Antibody (VS3-FY2908) (VS3-FY2908)
-
- Species Reactivity: Mouse
- Type: Mouse IgG
- Application: WB
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Cancer-related genes, Disease related genes, Human disease related genes, Plasma proteins
Intracellular, Membrane (different isoforms)
Cell type enhanced (Late spermatids)
Low immune cell specificity
Low cell line specificity
Probably forms a complex composed of chaperones HSP90 and HSP70, co-chaperones STIP1/HOP, CDC37, PPP5C, PTGES3/p23, TSC1 and client protein TSC2 (PubMed:29127155). Forms a complex composed of chaperones HSP90 and HSP70, co-chaperones CDC37, PPP5C, TSC1 and client protein TSC2, CDK4, AKT, RAF1 and NR3C1; this complex does not contain co-chaperones STIP1/HOP and PTGES3/p23 (PubMed:29127155). Forms a complex containing HSP90AA1, TSC1 and TSC2; TSC1 is required to recruit TCS2 to the complex (PubMed:29127155). Interacts (via C-terminus) with the closed form of HSP90AA1 (via the middle domain and TPR repeat-binding motif) (PubMed:29127155). Interacts with TSC2; the interaction stabilizes TSC2 and prevents TSC2 self-aggregation (PubMed:10585443, PubMed:15963462, PubMed:16464865, PubMed:9580671, PubMed:9809973, PubMed:29127155, PubMed:28215400). Interacts with DOCK7 (PubMed:15963462). Interacts with FBXW5 (PubMed:18381890). Interacts with TBC1D7 (PubMed:17658474). Interacts with WDR45B (PubMed:28561066). Interacts with RPAP3 and URI1 (PubMed:28561026).
Chaperone

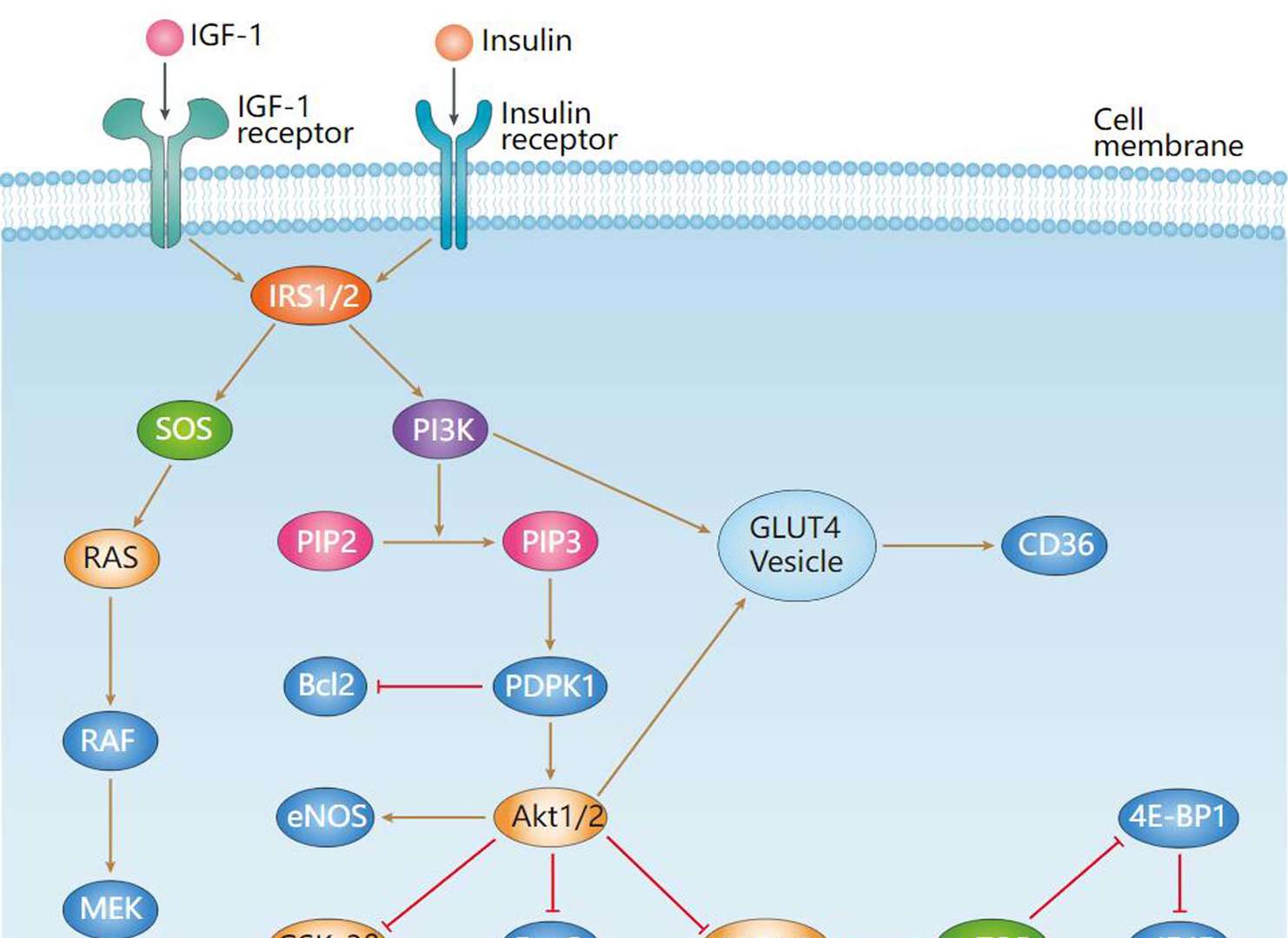 Insulin Signaling Pathway
Insulin Signaling Pathway
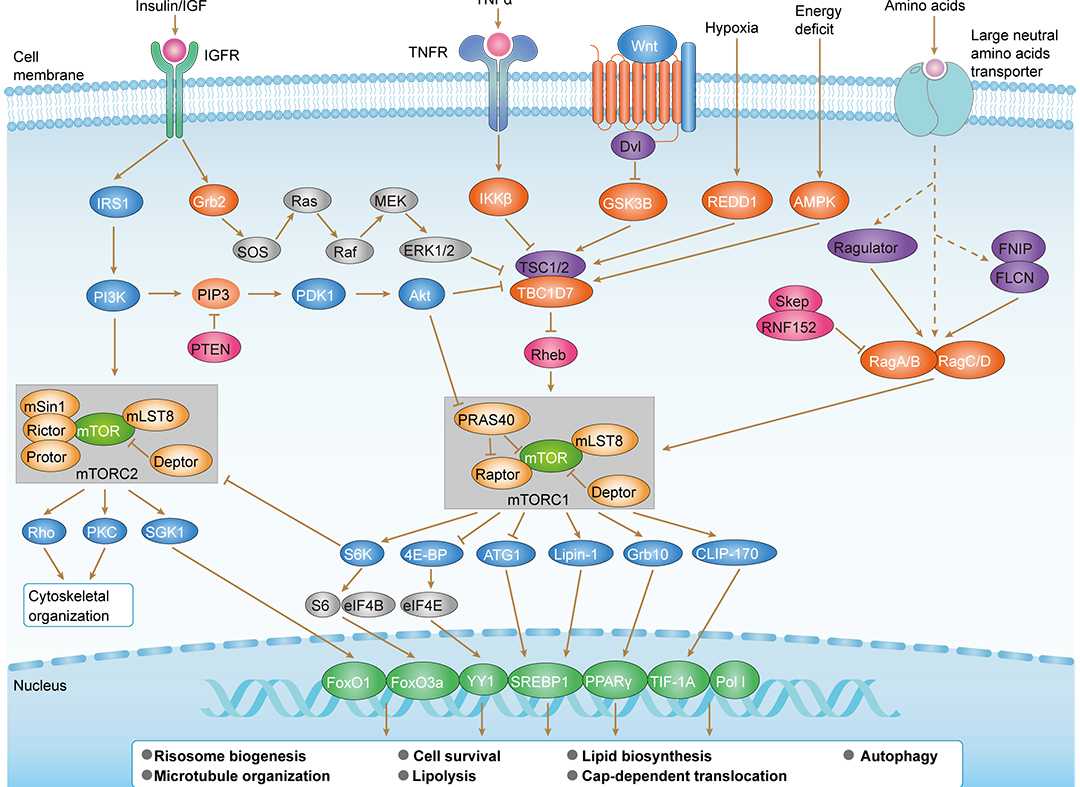 mTOR Signaling Pathway
mTOR Signaling Pathway
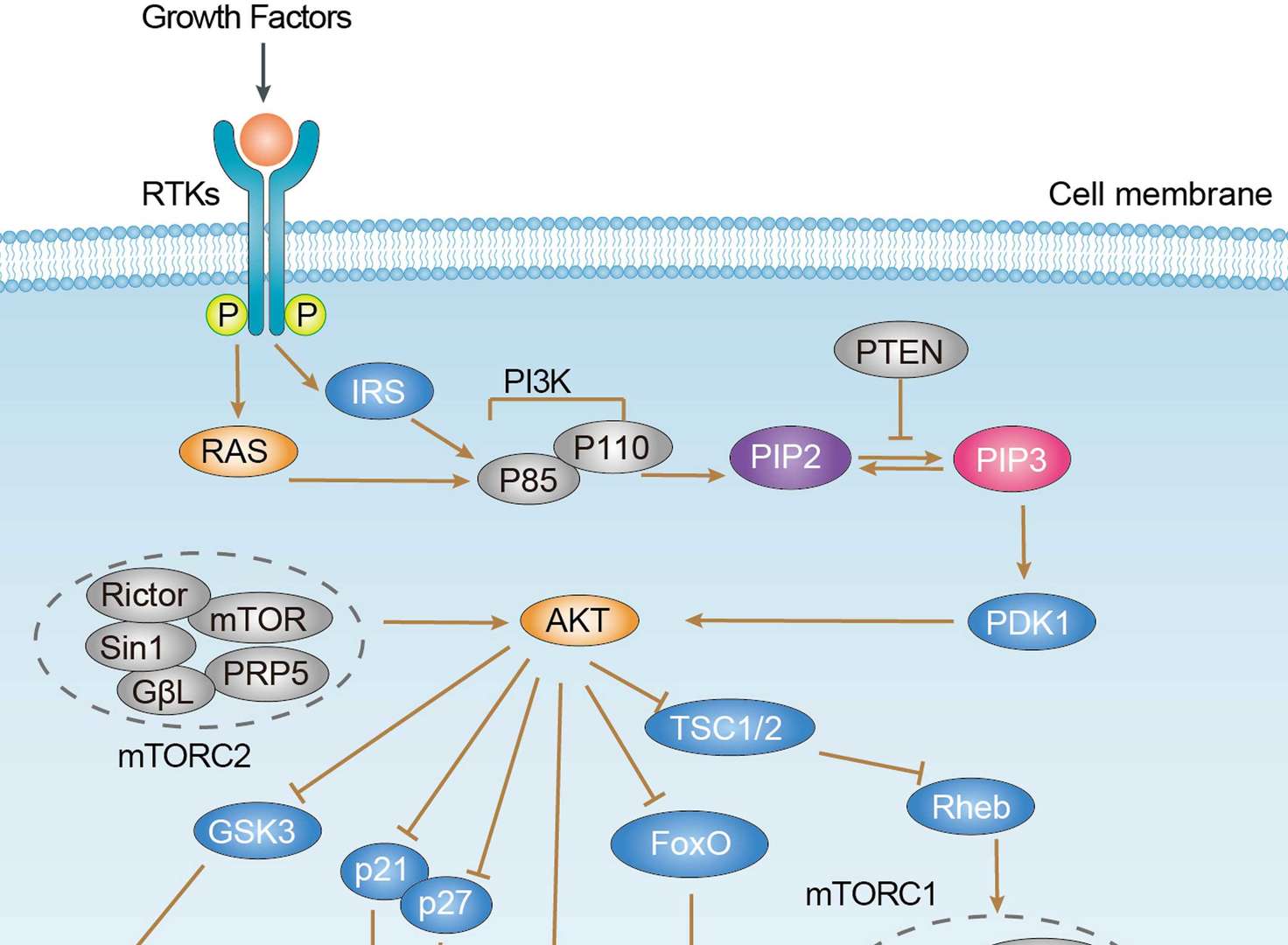 PI3K-Akt Signaling Pathway
PI3K-Akt Signaling Pathway
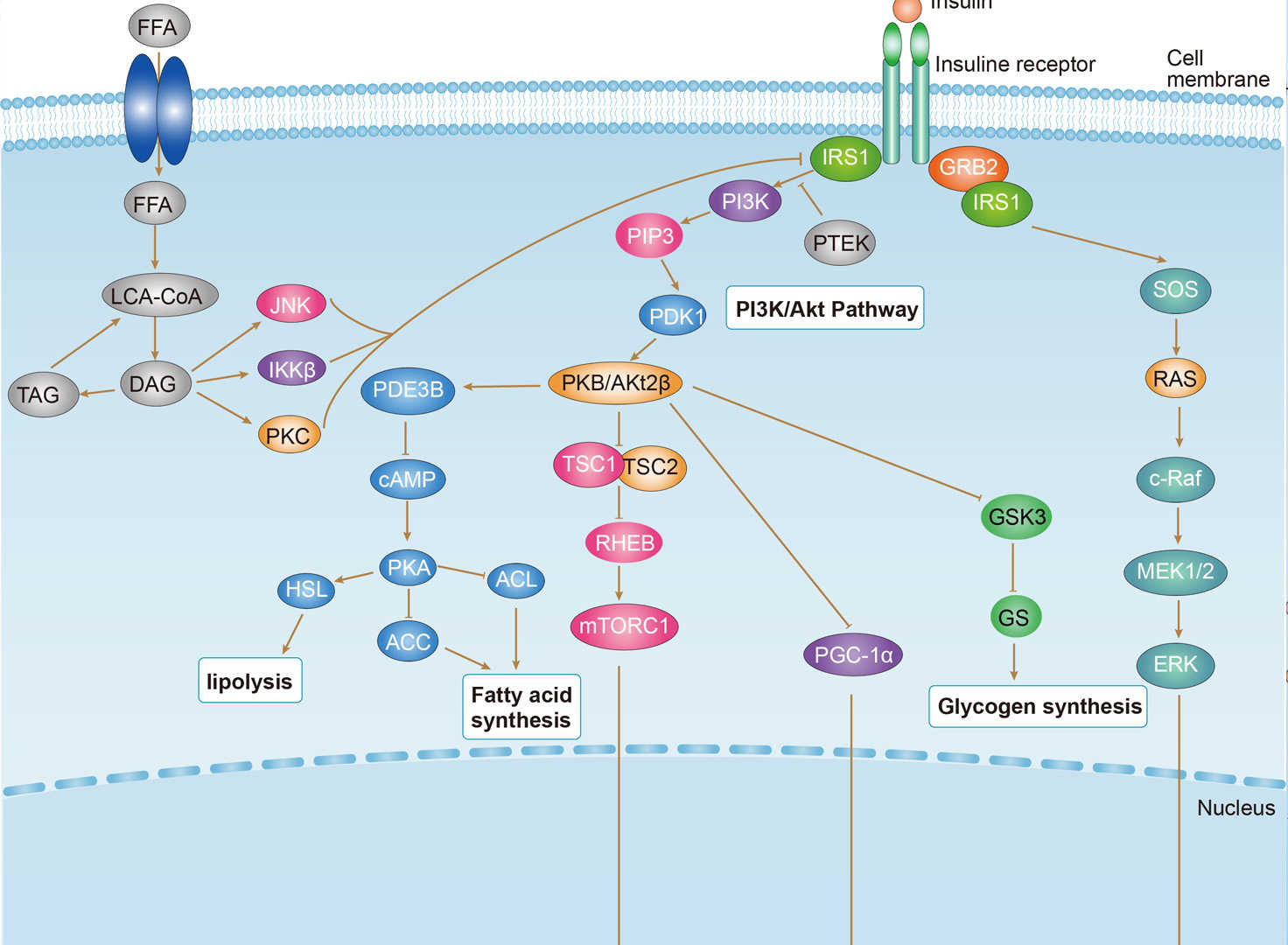 Insulin Resistance
Insulin Resistance