 Loading...
Loading...

CLDN18.2
Anti-CLDN18.2 Recombinant Antibody Products
- Human Anti-CLDN18.2 Recombinant Antibody (HPAB-AP289-YC) (HPAB-AP289-YC)
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) IgG1, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-AP283-YC) (HPAB-AP283-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG1, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-0120-YJ) (HPAB-0120-YJ)
-
- Species Reactivity: Human
- Type: Mouse IgG, κ
- Application: ELISA, WB, IHC-P
- Rabbit Anti-CLDN18.2 Recombinant Antibody (VS-0723-WK225) (VS-0723-WK225)
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, FC
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-0121-YJ) (HPAB-0121-YJ)
-
- Species Reactivity: Human
- Type: Mouse IgG, κ
- Application: ELISA, WB, IHC-P
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-AP285-YC) (HPAB-AP285-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG2a, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-AP284-YC) (HPAB-AP284-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG2a, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-AP282-YC) (HPAB-AP282-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG3, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-AP281-YC) (HPAB-AP281-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG3, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody (HPAB-AP280-YC) (HPAB-AP280-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG2a, κ
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody (HPAB-AP291-YC) (HPAB-AP291-YC)
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) IgG1, κ
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody (HPAB-AP290-YC) (HPAB-AP290-YC)
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) IgG1, κ
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody (HPAB-AP288-YC) (HPAB-AP288-YC)
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) IgG1, κ
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody (HPAB-AP287-YC) (HPAB-AP287-YC)
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) IgG1, κ
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody (HPAB-AP286-YC) (HPAB-AP286-YC)
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) IgG1, κ
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP285-YC-S(P)) (HPAB-AP285-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP284-YC-S(P)) (HPAB-AP284-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP283-YC-S(P)) (HPAB-AP283-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP282-YC-S(P)) (HPAB-AP282-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP281-YC-S(P)) (HPAB-AP281-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP280-YC-S(P)) (HPAB-AP280-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP291-YC-S(P)) (HPAB-AP291-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP290-YC-S(P)) (HPAB-AP290-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP289-YC-S(P)) (HPAB-AP289-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP288-YC-S(P)) (HPAB-AP288-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP287-YC-S(P)) (HPAB-AP287-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-AP286-YC-S(P)) (HPAB-AP286-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-0121-YJ-S(P)) (HPAB-0121-YJ-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA, WB, IHC-P
- Mouse Anti-CLDN18.2 Recombinant Antibody; scFv Fragment (HPAB-0120-YJ-S(P)) (HPAB-0120-YJ-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA, WB, IHC-P
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-0121-YJ-F(E)) (HPAB-0121-YJ-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA, WB, IHC-P
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-0120-YJ-F(E)) (HPAB-0120-YJ-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA, WB, IHC-P
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP285-YC-F(E)) (HPAB-AP285-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP284-YC-F(E)) (HPAB-AP284-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP283-YC-F(E)) (HPAB-AP283-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP282-YC-F(E)) (HPAB-AP282-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP281-YC-F(E)) (HPAB-AP281-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP280-YC-F(E)) (HPAB-AP280-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP291-YC-F(E)) (HPAB-AP291-YC-F(E))
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) Fab
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP290-YC-F(E)) (HPAB-AP290-YC-F(E))
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) Fab
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP289-YC-F(E)) (HPAB-AP289-YC-F(E))
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) Fab
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP288-YC-F(E)) (HPAB-AP288-YC-F(E))
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) Fab
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP287-YC-F(E)) (HPAB-AP287-YC-F(E))
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) Fab
- Application: ELISA
- Human Anti-CLDN18.2 Recombinant Antibody; Fab Fragment (HPAB-AP286-YC-F(E)) (HPAB-AP286-YC-F(E))
-
- Derivation: Chimeric (Mouse/Human)
- Species Reactivity: Human
- Type: Chimeric (Mouse/Human) Fab
- Application: ELISA
- Human Anti-CLDN18.2 scFv-Fc Chimera (VS-0325-FY88) (VS-0325-FY88)
-
- Species Reactivity: Human
- Type: Human IgG1, scFv-Fc
- Application: FC
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Creative Biolabs furnishes top-grade and all-inclusive recombinant antibodies specific to CLDN18.2. These antibodies are custom-tailored to fulfill the demands of biomedical researchers and institutions. With painstaking development, they ensure reliable functionality, thereby assisting researchers in advancing cancer research and immunotherapy endeavors.
CLDN18.2: A Critical Protein Target for Cancer Immunotherapy
CLDN18.2, which is a tight junction protein, is mainly found in the stomach and several cancer types such as gastric and pancreatic cancers. As part of the Claudin family, it has a crucial function in preserving the integrity of cell-cell connections and modulating epithelial cell function. The fact that it is overexpressed in malignant cells has turned CLDN18.2 into an attractive target for cancer immunotherapy. Antibodies directed against CLDN18.2 are under investigation as prospective treatments, particularly in targeted therapies like antibody-drug conjugates (ADCs), to selectively eradicate cancer cells while reducing harm to healthy tissues.
Alternative Names
Claudin 18.2
Anti-CLDN18.2 rAb Products
The state-of-the-art anti-CLDN18.2 recombinant antibodies we offer are meticulously engineered to reinforce and expedite investigations focusing on the development of CLDN18.2-targeted drugs. By supplying high-caliber and trustworthy products, we strive to drive scientific advancement and contribute to the discovery of novel therapies related to CLDN18.2, with a particular emphasis on cancer treatment.
| Cat. No. | Product Name | Target Species | Host Species | Applications |
| HPAB-AP289-YC | Human Anti-CLDN18.2 Recombinant Antibody | Human | Human | ELISA |
| HPAB-AP283-YC | Mouse Anti-CLDN18.2 Recombinant Antibody | Human | Mouse | ELISA |
| HPAB-0120-YJ | Mouse Anti-CLDN18.2 Recombinant Antibody | Human | Mouse | ELISA; WB; IHC-P |
| HPAB-0121-YJ | Mouse Anti-CLDN18.2 Recombinant Antibody | Human | Mouse | ELISA; WB; IHC-P |
| HPAB-AP284-YC | Mouse Anti-CLDN18.2 Recombinant Antibody | Human | Mouse | ELISA |
Creative Quality Control
To maintain the highest possible antibody quality benchmarks, we have instituted a comprehensive quality management framework. This framework secures the consistency and reliability of our products, allowing researchers to execute their work with confidence and competence.
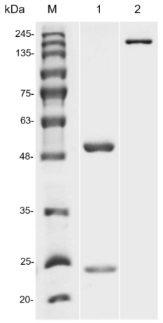 Fig.1 SDS-PAGE analysis of anti-CLDN18.2 antibody
Fig.1 SDS-PAGE analysis of anti-CLDN18.2 antibody
(Cat# HPAB-0120-YJ, Creative Biolabs).
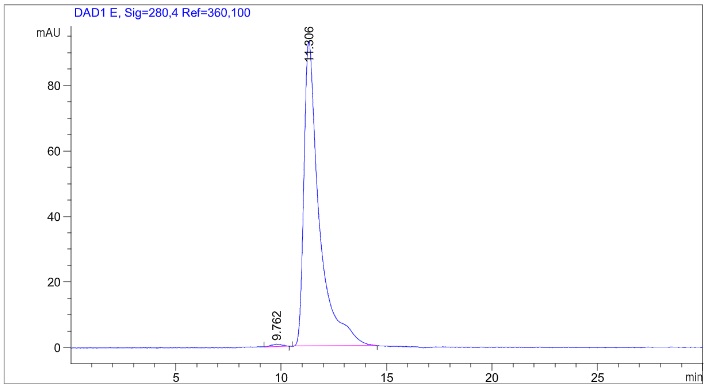 Fig.2 SEC-HPLC analysis of anti-CLDN18.2 antibody
Fig.2 SEC-HPLC analysis of anti-CLDN18.2 antibody
(Cat# HPAB-0120-YJ, Creative Biolabs).
Customer Reviews

Human Anti-CLDN18.2 Recombinant Antibody (Cat#: HPAB-AP289-YC)

Mouse Anti-CLDN18.2 Recombinant Antibody (Cat#: HPAB-0120-YJ)
rAb Production
A rich reservoir of experience is held by us in optimizing key processes such as expression vector design, transfected cell line screening, and antibody purification. Our services, which are comprehensive in nature, cover the entire spectrum from gene synthesis right through to antibody production, ensuring reliable and efficient solutions are at the disposal of biomedical researchers.
Featured Anti-CLDN18.2 Recombinant Antibody Production Platforms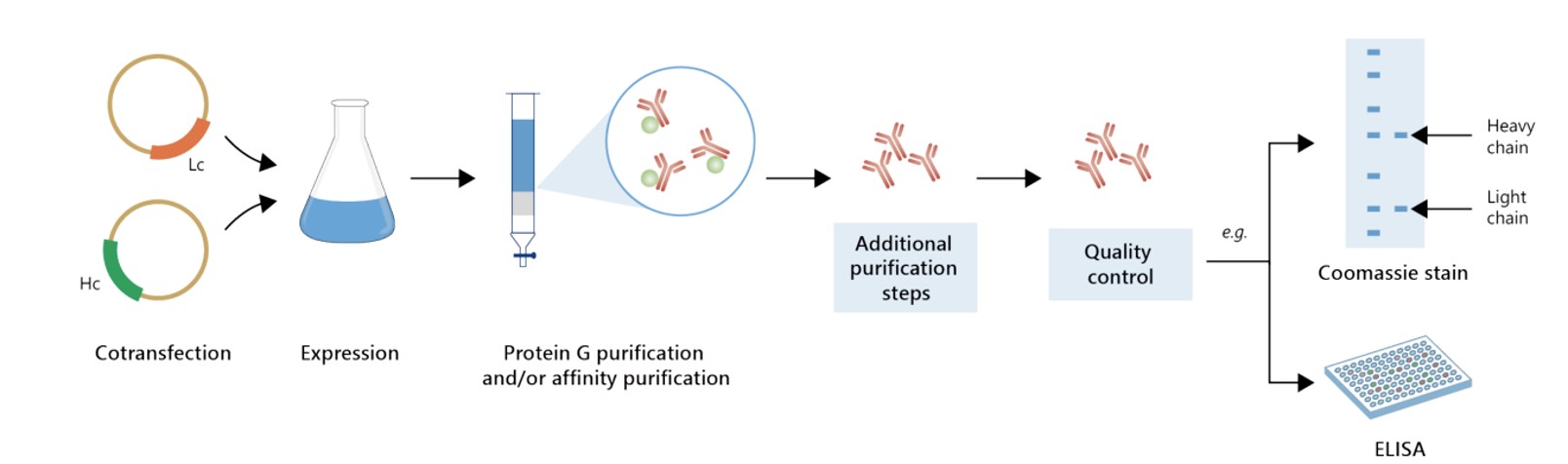
Fig.3 Milligram-scale recombinant antibody production.
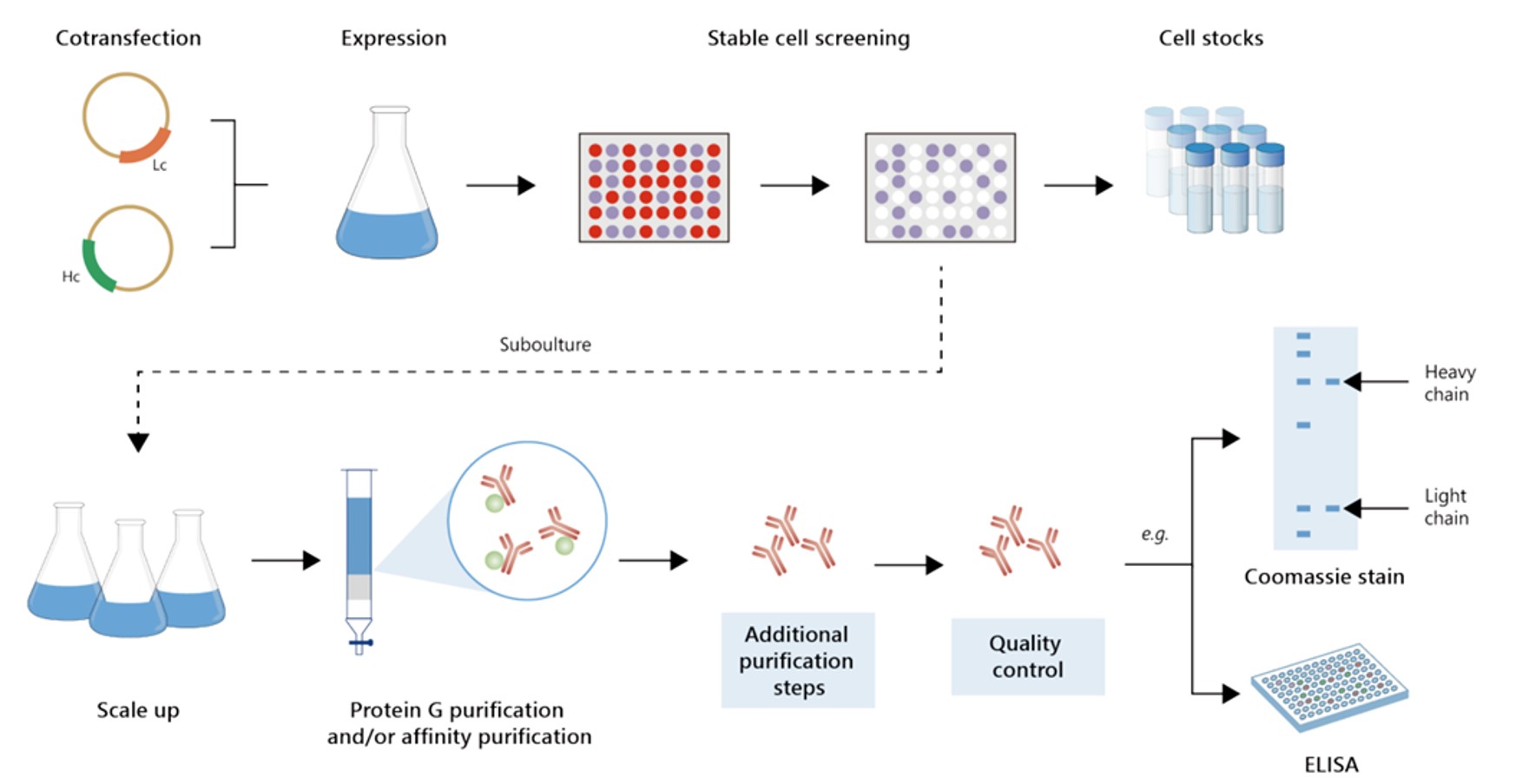 Fig.4 Gram-scale recombinant antibody production.
Fig.4 Gram-scale recombinant antibody production.
rAb Modalities
Creative Biolabs devotes itself to furnishing researchers with high-quality recombinant antibodies in diverse formats. These formats, including full-length antibodies, Fab fragments, scFv, scFv-Fc and so on, are customized to meet a wide array of research needs. The variety ensures flexibility and broad applicability in experimental operations.
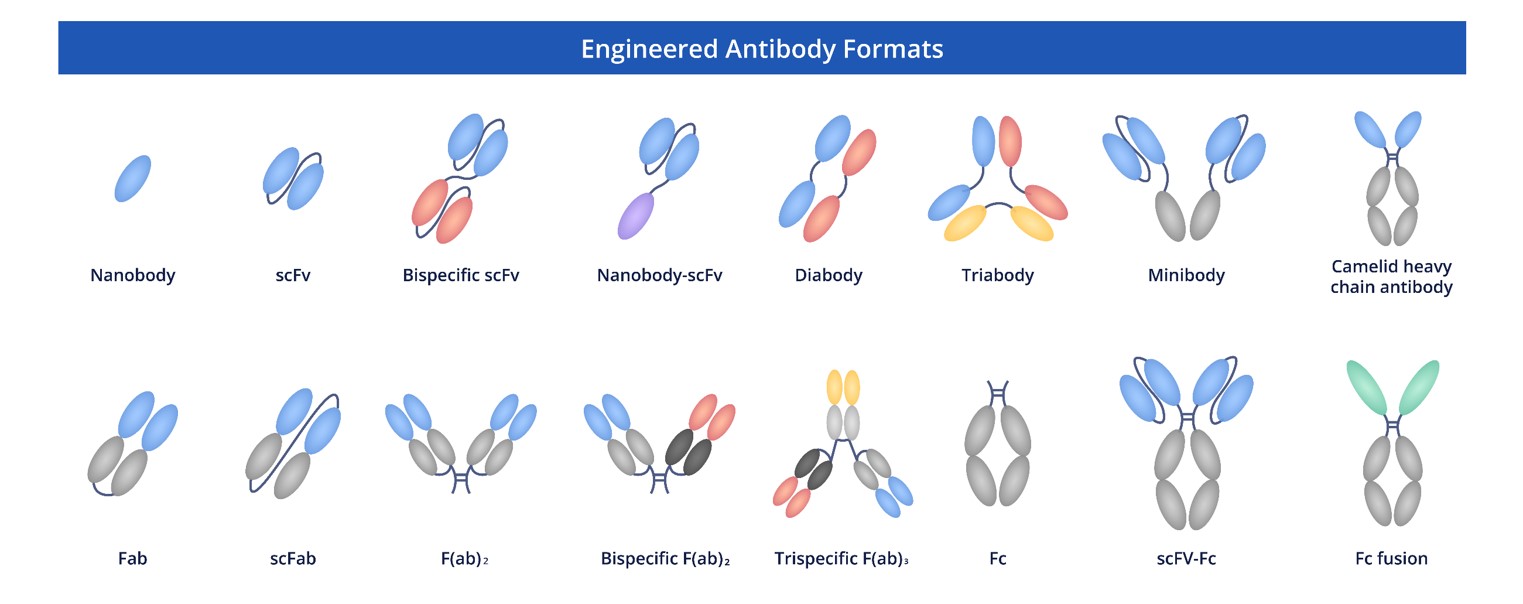 Fig.5 Full Length Anti-CLDN18.2 Recombinant Antibody Production and Modalities.
Fig.5 Full Length Anti-CLDN18.2 Recombinant Antibody Production and Modalities.
Drug Information Targeting CLDN18.2
Table 1. Therapeutic approaches targeting CLDN18.2 in clinical development.
| Research phase | Company | Classification | Indications | Details |
| Launched - 2024 | Astellas Pharma | Chimeric Monoclonal Antibody | Cancer; Digestive-gastrointestinal cancer | This is a monoclonal IgG1 antibody against gastric cell surface protein GC182 (CLDN18.2) developed by Astellas Pharma. In 2024, the product was approved and launched in Japan for the treatment of patients with CLDN18.2 positive, unresectable, advanced or recurrent gastric cancer. Regulatory review is ongoing in the U.S. and the E.U. for the first-line treatment of CLDN18.2-positive, HER2-negative, metastatic adenocarcinomas of the stomach or the gastroesophageal junction. |
| Phase II | MabSpace Biosciences | Humanized Monoclonal Antibody | Cancer | It is a humanized low-fucose monoclonal antibody targeting human Claudin18 isoform 2 (CLDN18.2) in phase II clinical trials at Transcenta (formerly known as MabSpace Biosciences) for the treatment of solid tumors expressing Claudin18.2 such as gastric, pancreatic and biliary tract cancers. |
| Phase I | ABL Bio | Monoclonal Antibody | Cancer | It is a claudin 18.2 x 4-1BB bispecific antibody jointly developed by ABL Bio and I-Mab Biopharma. A phase I clinical trial is ongoing for the treatment of patients with advanced solid tumors, including gastric cancer, gastroesophageal junction carcinoma, esophageal adenocarcinoma, and pancreatic ductal carcinoma. |
If you would like further details about our anti-CLDN18.2 recombinant antibody products, feel free to contact us. We look forward to assisting you and exploring the potential for a productive and mutually beneficial partnership.
