Glycoengineering based IgA Half-Life Extension Services
Optimizing Glycan Architecture to Enhance IgA Therapeutic Durability
IgA antibodies, especially IgA1 and IgA2 subclasses, represent an emerging class of therapeutic candidates due to their natural role in mucosal immunity, unique effector functions, and potential advantages in immune modulation. However, their therapeutic application in systemic contexts is limited by their short serum half-life—a limitation that stems not only from poor FcRn engagement but also from their complex glycosylation profiles, which promote rapid clearance by hepatic lectin receptors such as the asialoglycoprotein receptor (ASGPR).
At Creative Biolabs, we offer specialized Glycoengineering-Based IgA Half-Life Extension Services that directly address this challenge by optimizing the glycan structures on IgA molecules to minimize rapid clearance and enhance in vivo stability. Our advanced glycosylation platform allows for precision control of both N- and O-linked glycans, ensuring optimal half-life without compromising antigen binding or receptor interactions.
What We Offer
We employ two synergistic strategies to modify glycosylation patterns on IgA molecules for enhanced serum retention:
IgA Terminal Sialylation Optimization Service
One of the key determinants of glycoprotein clearance is the presence—or absence—of terminal sialic acids on N- and O-linked glycans. IgA molecules with exposed galactose or N-acetylglucosamine (GlcNAc) residues are rapidly recognized and removed by ASGPR in the liver. Increasing terminal α2,6- or α2,3-sialylation shields these recognition sites and improves pharmacokinetics.
Service Highlights:
- Host Cell Engineering: Use of CHO or HEK293 lines overexpressing specific sialyltransferases (e.g., ST6GAL1) to maximize terminal sialylation.
- In Vitro Enzymatic Remodeling: Application of purified sialyltransferases to remodel glycan termini post-expression.
- Sialic Acid Linkage Control: Tailored addition of α2,6- or α2,3-linkages based on the target indication and receptor interactions.
- Glycan Profiling: LC-MS/MS, HPAEC-PAD, and MALDI-TOF analysis to quantify sialylation efficiency and uniformity.
Click the key words to learn more!
IgA ASGPR Clearance Evasion Service
ASGPR-mediated endocytosis is a major pathway for the clearance of desialylated glycoproteins, especially those with terminal galactose residues. Our clearance evasion strategy involves both direct glycan masking and cell line selection to minimize ASGPR recognition.
Service Highlights:
- Glycan Remodeling: Removal of terminal galactose via glycosidase treatment or prevention of galactosylation through glycosyltransferase knockdown (e.g., B4GALT1).
- Custom Host Cell Selection: Use of engineered cell lines with low galactosylation or high branching/sialylation activity.
- ASGPR Binding Assays: In vitro receptor-binding assays to quantify interaction with ASGPR using fluorescent or SPR-based platforms.
- In Vivo Validation: Optional PK testing in animal models with ASGPR-dependent clearance mechanisms.
Click the key words to learn more!
Why Glycoengineering Matters for IgA Therapeutics
IgA's glycosylation profile is one of its most distinguishing and functionally relevant characteristics—but it can also be a liability in therapeutic contexts. Native IgA1 contains multiple O-linked glycans in its hinge region and up to five N-linked glycans, all of which can contribute to variable pharmacokinetics. Glycoengineering addresses this bottleneck, providing a rational and reproducible method for extending half-life while improving drug-like properties.
- Long-acting mucosal immunotherapies
- IgA checkpoint inhibitors for oncology
- Anti-infective antibodies with systemic persistence
- IgA-Fc fusion constructs with improved bioavailability
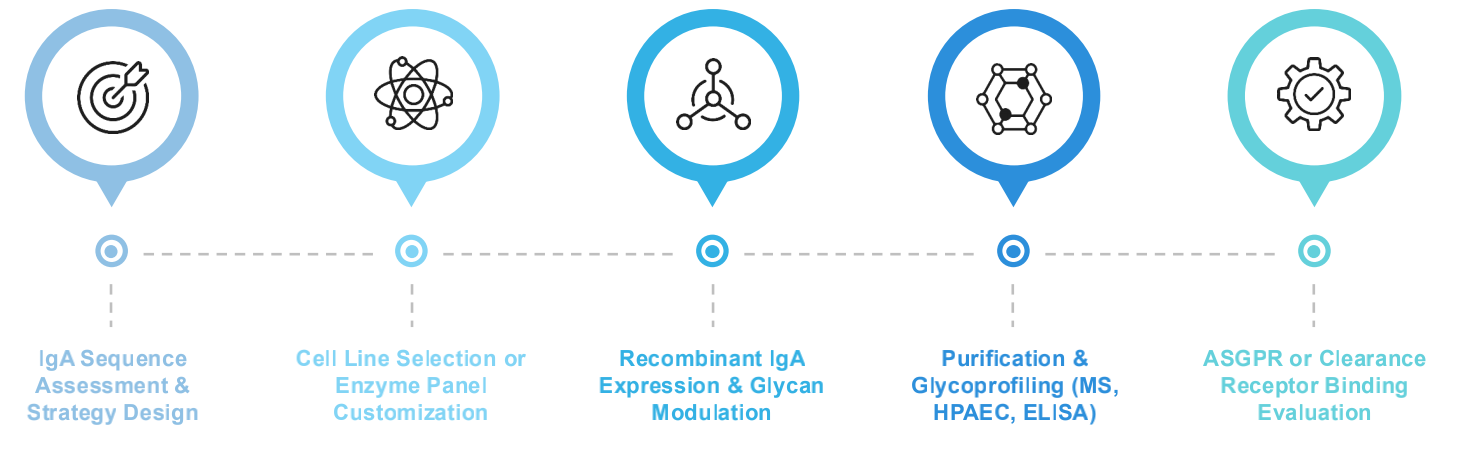
Service Workflow
Partner with Creative Biolabs
With deep expertise in glycosylation biology, antibody expression, and immunoglobulin engineering, Creative Biolabs provides the precision tools and platforms necessary to unlock the full therapeutic potential of IgA. Whether you are improving an existing lead or building a novel therapeutic from the ground up, our Glycoengineering-Based IgA Half-Life Extension Services offer powerful, evidence-based solutions to extend half-life, reduce immunogenicity, and enhance bioavailability.
Contact us today to discover how our glycoengineering expertise can advance your IgA program toward success in preclinical development.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



