Lysine-based Conjugation Service
Creative Biolabs can perform lysine-based conjugation in ADC development. Scientists from Creative Biolabs have specialized experience in providing antibody-drug conjugates (ADCs) suitable for commercially-available, cost-effective and large-scale manufacturing.
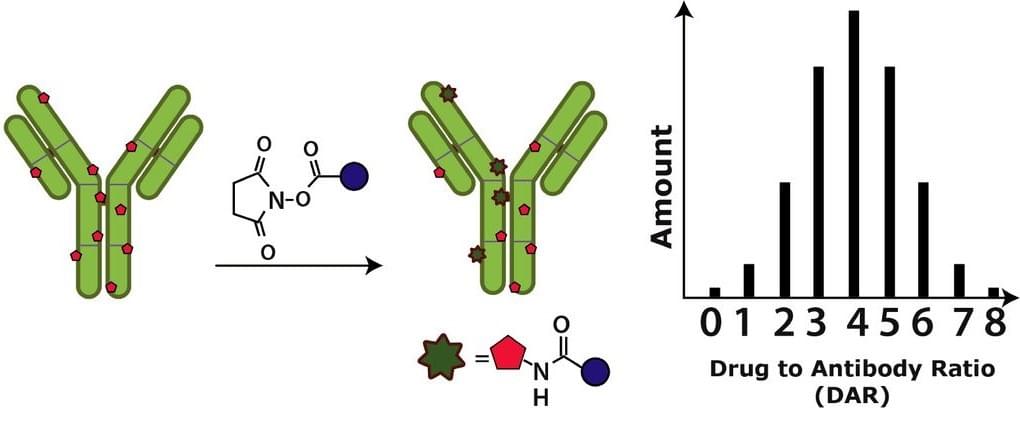 Fig.1 Formation of lysine-based conjugation.1
Fig.1 Formation of lysine-based conjugation.1
In ADC development, lysine-based conjugation is one of the most widely used non-specific conjugation strategies. So far, the conjugation technology through lysine side chain amines has proceeded to clinical trials and conjugate payloads such as DM1, DM4 or calicheamicin with mAbs. The side-chain amino groups of lysine residues are good nucleophiles, so lysine residues exposed at the surface of antibodies, in areas of structural flexibility, have large solvent accessibility and can be used as sites for drug conjugation. There are approximately 80 reactive lysines in an IgG scaffold and among which, over 20 resides are potential candidates for chemical elaboration. In this strategy, linkers bearing activated esters, usually O-succinimide reagents like N-hydroxysuccinimidyl (NHS) or sulfo-NHS esters and imido ester compounds like Traut’s reagent, react with solvent-accessible lysine residues to form amide or amidine bonds. As a result, heterogeneous mixtures of ADCs with various DAR and conjugation positions are available. Usually, the methods of lysine conjugation include one-step and two-step conjugation. In one-step conjugation, the lysine ε-amino groups of an antibody react with an amine-reactive group of a drug to generate amide bonds. While the two-step conjugation first proceeds with introducing chemical functionalities to modify the lysine resides of an antibody. The modified antibody with new functionalities can subsequently react with the specific reactive groups of a drug. For instance, the moieties of maleimido, pyridyldisulfide or iodoacetamido can be introduced with O-succinimide reagents. And usually the reactive group of the drug is a sulfhydryl moiety and the conjugation of the drug to the antibody is similar.
The strategy of lysine-based conjugation can be applied to various cytotoxic agents. Moreover, site-specific lysine conjugation is also developed based on azetidinone chemistry.
Based on the full understanding of your individual requirements and unrivaled expertise in ADC development, scientists and engineers from Creative Biolabs can design and optimize conjugation strategies to facilitate your researches about ADCs.
There are various strategies used for antibody modification and conjugation:
- Cysteine-based conjugation
- Lysine-based conjugation
- Carbohydrate-based conjugation
- Unnatural amino acids-based conjugation
- Thio-engineered antibody
- Enzymatic modified antibody
- UV photocrosslinking(UV-NBS)
- Meditope-enabled antibodies
Reference
- Peters, Christina, and Stuart Brown. "Antibody–drug conjugates as novel anti-cancer chemotherapeutics." Bioscience reports 35.4 (2015): e00225. Distributed under Open Access license CC BY 3.0. The image was modified by extracting and using only part of the original image.
For research use only. Not intended for any clinical use.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.



