Anti-NT5E Recombinant Antibody Products
 Loading...
Loading...Anti-NT5E Products
 Loading...
Loading...- Human Anti-NT5E Recombinant Antibody (PABJ-0226) (PABJ-0226)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, Block
- Human Anti-NT5E Recombinant Antibody (TAB-600MZ) (TAB-600MZ)
-
- Derivation: Human phage display library
- Species Reactivity: Murine, cynomolgus
- Type: Human IgG1
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-599MZ-F(E)) (TAB-599MZ-F(E))
-
- Derivation: Human phage display library
- Species Reactivity: HUman, Mouse, Monkey
- Type: Human Fab
- Application: FC
- Human Anti-NT5E Recombinant Antibody (HPAB-N0196-YC) (HPAB-N0196-YC)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Mouse Anti-NT5E Recombinant Antibody (HPAB-0500-FY) (HPAB-0500-FY)
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Mouse IgG
- Application: Neut, FC, Block, FuncS, WB
- Mouse Anti-NT5E Recombinant Antibody (HPAB-0501-FY) (HPAB-0501-FY)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: Neut, FC, Block, FuncS, WB
- Mouse Anti-NT5E Recombinant Antibody (HPAB-0502-FY) (HPAB-0502-FY)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: Neut, FC, Block, FuncS, Inhib
- Recombinant Rat Anti-NT5E Antibody (TY/11.8) (FN-212CQ)
-
- Species Reactivity: Mouse
- Type: Rat IgG1
- Application: FC, FuncS
- Rabbit Anti-NT5E Antibody, mRNA (TAB-693CL-mRNA)
-
- Species Reactivity: Human
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, IHC
- Rabbit Anti-Nt5e Recombinant Antibody (clone YDBYT 208.3) (FAMAB-1684CQ)
-
- Species Reactivity: Mouse
- Type: Rabbit IgG
- Application: ELISA
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: ELISA, IHC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-600MZ-F(E)) (TAB-600MZ-F(E))
-
- Derivation: Human phage display library
- Species Reactivity: Murine, cynomolgus
- Type: Human Fab
- Application: FC
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: WB, IHC
-
- Species Reactivity: Human
- Type: ADCC enhanced antibody
- Human Anti-NT5E Recombinant Antibody (TAB-593MZ) (TAB-593MZ)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-NT5E Recombinant Antibody (TAB-594MZ) (TAB-594MZ)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-NT5E Recombinant Antibody (TAB-595MZ) (TAB-595MZ)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC, FuncS
- Human Anti-NT5E Recombinant Antibody (TAB-596MZ) (TAB-596MZ)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-NT5E Recombinant Antibody (TAB-597MZ) (TAB-597MZ)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-NT5E Recombinant Antibody (TAB-598MZ) (TAB-598MZ)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-593MZ-S(P)) (TAB-593MZ-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-594MZ-S(P)) (TAB-594MZ-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-595MZ-S(P)) (TAB-595MZ-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-596MZ-S(P)) (TAB-596MZ-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-597MZ-S(P)) (TAB-597MZ-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-593MZ-F(E)) (TAB-593MZ-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-594MZ-F(E)) (TAB-594MZ-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-595MZ-F(E)) (TAB-595MZ-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-596MZ-F(E)) (TAB-596MZ-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-597MZ-F(E)) (TAB-597MZ-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (TAB-598MZ-F(E)) (TAB-598MZ-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: ICC, WB
- Recombinant Rat Anti-Nt5e Antibody (TY/23) (NEUT-1800CQ)
-
- Species Reactivity: Mouse
- Type: IgG2a
- Application: BL
- Recombinant Mouse Anti-NT5E Antibody (NEUT-1799CQ) (NEUT-1799CQ)
-
- Species Reactivity: Human
- Type: IgG2a, κ
- Application: FC, IHC-Fr, IF, IP, Inhib
- Anti-Human NT5E Recombinant Antibody (TAB-441CQ) (TAB-441CQ)
-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG1, λ
- Application: ELISA, IHC, FC, IP, IF, FuncS
- Mouse Anti-NT5E Recombinant Antibody (HPAB-N0374-YC) (HPAB-N0374-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA, FC, Inhib
- Human Anti-NT5E Recombinant Antibody (HPAB-N0375-YC) (HPAB-N0375-YC)
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, FC, Inhib
- Mouse Anti-NT5E Recombinant Antibody (MOB-1936z) (MOB-1936z)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, IHC, IF
- Rabbit Anti-NT5E Polyclonal Antibody (MRO-1759-CN) (MRO-1759-CN)
-
- Species Reactivity: Human, Mouse
- Type: Rabbit IgG
- Application: WB, IF, IHC, FC
- Afuco™ Anti-NT5E ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-441CQ)
-
- Derivation: Human
- Species Reactivity: Human
- Type: ADCC enhanced antibody
- Application: ELISA, IHC, FC, IP, IF, FuncS
- Mouse Anti-NT5E Recombinant Antibody (HPAB-M0172-YC) (HPAB-M0172-YC)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: FuncS
- Rabbit Anti-Nt5e Antibody (clone YDBYT 208.3), mRNA (FAMAB-1684CQ-mRNA)
-
- Species Reactivity: Mouse
- Mouse Anti-NT5E Recombinant Antibody (clone 7H4-H0) (VS3-XY1186)
-
- Species Reactivity: Human
- Type: Mouse IgG1
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Mouse Anti-NT5E Recombinant Antibody (clone 4G6B10) (VS3-XY1188)
-
- Type: Mouse IgG1
- Application: IHC
- Mouse Anti-NT5E Recombinant Antibody (clone 4G6E3) (VS3-XY1189)
-
- Type: Mouse IgG1
- Application: IHC
- Mouse Anti-NT5E Recombinant Antibody (clone 1D7) (VS3-XY1190)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, IHC
- Mouse Anti-NT5E Recombinant Antibody (clone 6H10) (VS3-XY1191)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB, IHC, FC
- AbPlus™ Anti-Nt5e Magnetic Beads (VS-0724-YC1442) (VS-0724-YC1442)
-
- Target: Nt5e
- Target Species: Rat
- Application: IP, Protein Purification
- AbPlus™ Anti-NT5E Magnetic Beads (VS-0724-YC1493) (VS-0724-YC1493)
-
- Target: NT5E
- Target Species: Human
- Application: IP, Protein Purification
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-598MZ-S(P)) (TAB-598MZ-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-599MZ-S(P)) (TAB-599MZ-S(P))
-
- Derivation: Human phage display library
- Species Reactivity: HUman, Mouse, Monkey
- Type: Human scFv
- Application: FC
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (TAB-600MZ-S(P)) (TAB-600MZ-S(P))
-
- Derivation: Human phage display library
- Species Reactivity: Murine, cynomolgus
- Type: Human scFv
- Application: FC
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: FC, Block
- Mouse Anti-NT5E Recombinant Antibody (HPAB-0503-FY) (HPAB-0503-FY)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: Neut, FC, Block, FuncS
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: IHC, ELISA
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-M0172-YC-F(E)) (HPAB-M0172-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: FC, Inhib
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-M0173-YC-F(E)) (HPAB-M0173-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: FC, FuncS
- Recombinant Anti-Human NT5E Antibody Fab Fragment (MOB-1936z-F(E))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Fab
- Application: FC, Neut, Funcs
- Recombinant Anti-Human NT5E Antibody scFv Fragment (MOB-1936z-S(P))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: scFv
- Application: WB, ELISA, IHC, FuncS
-
- Species Reactivity: Human
- Type: Antibody
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-N0374-YC-S(P)) (HPAB-N0374-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA, FC, Inhib
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-N0375-YC-S(P)) (HPAB-N0375-YC-S(P))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, FC, Inhib
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-M0172-YC-S(P)) (HPAB-M0172-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: FC, Inhib
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-M0173-YC-S(P)) (HPAB-M0173-YC-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: FC, FuncS
- Human Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-N0196-YC-S(P)) (HPAB-N0196-YC-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: FC
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-N0374-YC-F(E)) (HPAB-N0374-YC-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA, FC, Inhib
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-N0375-YC-F(E)) (HPAB-N0375-YC-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, FC, Inhib
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-0503-FY-F(E)) (HPAB-0503-FY-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: Neut, FC
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-0501-FY-F(E)) (HPAB-0501-FY-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: Neut, FC
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-0502-FY-F(E)) (HPAB-0502-FY-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: Neut, FC
- Mouse Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-0500-FY-F(E)) (HPAB-0500-FY-F(E))
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Mouse Fab
- Application: Neut, FC
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-0503-FY-S(P)) (HPAB-0503-FY-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: Neut, FC
- Human Anti-NT5E Recombinant Antibody; Fab Fragment (HPAB-N0196-YC-F(E)) (HPAB-N0196-YC-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-0502-FY-S(P)) (HPAB-0502-FY-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: Neut, FC
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-0500-FY-S(P)) (HPAB-0500-FY-S(P))
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Mouse scFv
- Application: Neut, FC
- Mouse Anti-NT5E Recombinant Antibody; scFv Fragment (HPAB-0501-FY-S(P)) (HPAB-0501-FY-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: Neut, FC
-
- Species Reactivity: Mouse, Cynomolgus
- Target: NT5E
- Host Animal: Human
- Application: ELISA, FC, Cell-uptake
- Anti-NT5E Immunohistochemistry Kit (VS-0525-XY5002)
-
- Species Reactivity: Human
- Target: NT5E
- Application: IHC
- Human Anti-NT5E (clone Uliledlimab) scFv-Fc Chimera (VS-0425-FY59)
-
- Species Reactivity: Human
- Type: Human IgG1, scFv-Fc
- Application: FC, ELISA
- Anti-Rat NT5E Immunohistochemistry Kit (VS-0525-XY5004)
-
- Species Reactivity: Human, Mouse, Rat
- Target: NT5E
- Application: IHC
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, IHC-P, ICC, IF, FC
- Anti-Mouse NT5E Immunohistochemistry Kit (VS-0525-XY5003)
-
- Species Reactivity: Human, Mouse, Rat, Bovine
- Target: NT5E
- Application: IHC
- Mouse Anti-NT5E Monoclonal Antibody (VS7-0425-WR510) (VS7-0425-WR510)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: IHC-P, IF, ELISA
-
- Species Reactivity: Human
- Type: Human IgG1 lambda
- Application: ELISA, IHC, FC, IP, IF
-
- Derivation: Phage display library screening
- Species Reactivity: Human
- Type: IgG
- Application: WB, IHC, ICC
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Creative Biolabs is committed to advancing research on the target NT5E by providing high-quality, cutting-edge recombinant antibodies designed specifically for this target. Leveraging innovative technology, our antibodies offer unparalleled reliability and performance, catering to the diverse needs of researchers globally. Through these premium products, we aim to support meaningful scientific discoveries and contribute to the progress of biomedical research, especially in areas exploring the vital functions of NT5E.
NT5E: A crucial cell surface enzyme in the immune disorders and cancer
NT5E (CD73) is a cell surface enzyme responsible for converting adenosine monophosphate (AMP) into adenosine, a molecule involved in regulating immune responses and inflammation. It plays a crucial role in creating an immunosuppressive microenvironment, particularly in cancer, by promoting immune tolerance and tumor progression. NT5E is overexpressed in various cancers and contributes to immune evasion by enhancing adenosine signaling. In addition to cancer, NT5E is implicated in autoimmune diseases and chronic inflammation. Due to its central role in immune modulation, NT5E has become a promising therapeutic target, with inhibitors being explored for cancer immunotherapy and other inflammatory disorders.
Alternative Names
NT; eN; NT5; NTE; eNT; CD73; E5NT; CALJA
Background
The protein encoded by this gene is a plasma membrane protein that catalyzes the conversion of extracellular nucleotides to membrane-permeable nucleosides. The encoded protein is used as a determinant of lymphocyte differentiation. Defects in this gene can lead to the calcification of joints and arteries. Two transcript variants encoding different isoforms have been found for this gene.
CD markers, Disease related genes, Enzymes, Human disease related genes, Metabolic proteins, Potential drug targets
Intracellular, Membrane (different isoforms)
Cell type enhanced (Pancreatic endocrine cells, Rod photoreceptor cells, Oligodendrocyte precursor cells, Glandular and luminal cells)
Immune cell enhanced (naive B-cell, naive CD8 T-cell)
Cell line enhanced (hTERT-RPE1, LHCN-M2, TIME, U-87 MG)
Homodimer.
Hydrolase
Anti-NT5E rAb Products
Creative Biolabs is dedicated to advancing research by providing high-quality anti-NT5E recombinant antibodies. Our products offer exceptional performance, reliable results, and outstanding technical support, empowering researchers to explore the role of NT5E in immune regulation and disease progression.
| Cat. No. | Product Name | Target Species | Host Species | Applications |
| TAB-441CQ | Anti-Human NT5E Recombinant Antibody (Oleclumab) | Human | Human | ELISA |
| PABJ-0226 | Human Anti-NT5E Recombinant Antibody (clone IPH5301) | Human | Human | ELISA; Block |
| HPAB-M0172-YC | Mouse Anti-NT5E Recombinant Antibody | Human | Mouse | FuncS |
| HPAB-N0375-YC | Human Anti-NT5E Recombinant Antibody | Human | Human | ELISA; FC; Inhib |
| HPAB-0500-FY | Mouse Anti-NT5E Recombinant Antibody | Human; Cynomolgus monkey | Mouse | Neut; FC; Block; FuncS; WB |
Creative Quality Control
We have developed a comprehensive quality management system to guarantee the superior quality of our products. This system is designed to not only meet but exceed our customers' expectations, enabling them to efficiently advance drug development processes and achieve their research goals with confidence.
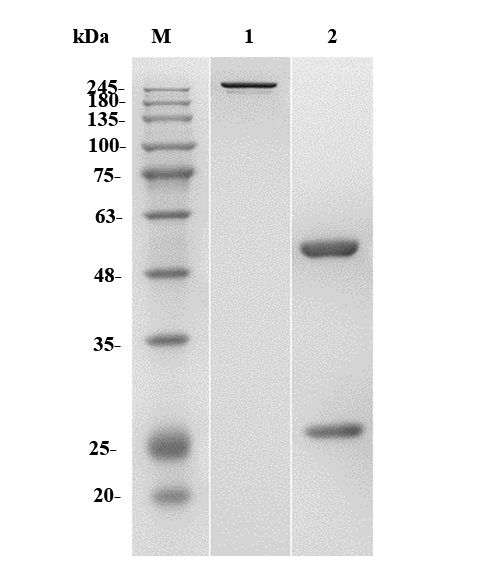 Fig.1 SDS-PAGE analysis of anti-NT5E antibody
Fig.1 SDS-PAGE analysis of anti-NT5E antibody
(Cat# PABJ-0226, Creative Biolabs).
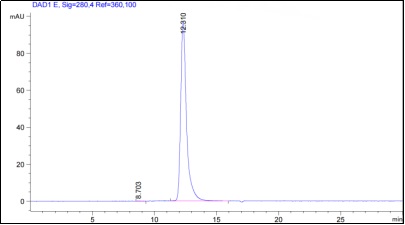 Fig.2 SEC-HPLC analysis of anti-NT5E antibody
Fig.2 SEC-HPLC analysis of anti-NT5E antibody
(Cat# PABJ-0226, Creative Biolabs).
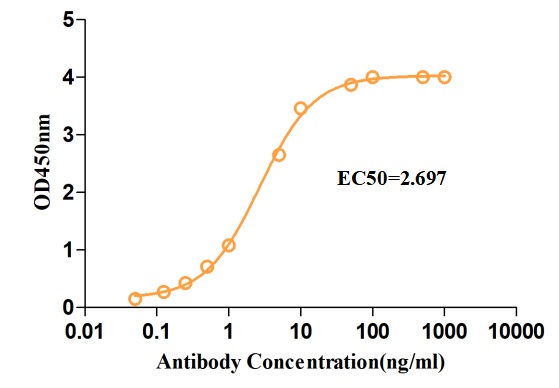 Fig.3 ELISA analysis of anti-NT5E antibody
Fig.3 ELISA analysis of anti-NT5E antibody
(Cat# PABJ-0226, Creative Biolabs).
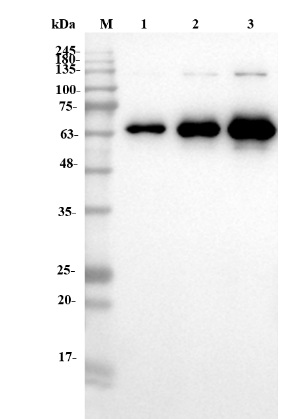 Fig.4 WB analysis of anti-NT5E antibody
Fig.4 WB analysis of anti-NT5E antibody
(Cat# PABJ-0226, Creative Biolabs).
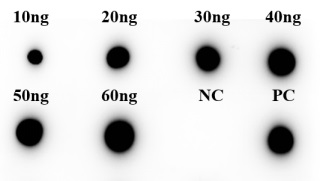 Fig.5 DB analysis of anti-NT5E antibody
Fig.5 DB analysis of anti-NT5E antibody
(Cat# PABJ-0226, Creative Biolabs).
Customer Reviews

Human Anti-NT5E Recombinant Antibody (clone IPH5301) (Cat#: PABJ-0226)

Human Anti-NT5E Recombinant Antibody (Cat#: HPAB-N0375-YC)

Mouse Anti-NT5E Recombinant Antibody (Cat#: HPAB-0500-FY)
rAb Production
Our comprehensive services span the entire process, from gene synthesis to the production of top-tier recombinant antibodies. This ensures a seamless experience for our customers, enabling them to focus on their research without concerns about quality or timelines. Leveraging our expertise, we provide tailored antibody production solutions to meet the specific needs of researchers, delivering optimal results.
Featured Anti-NT5E Recombinant Antibody Production Platforms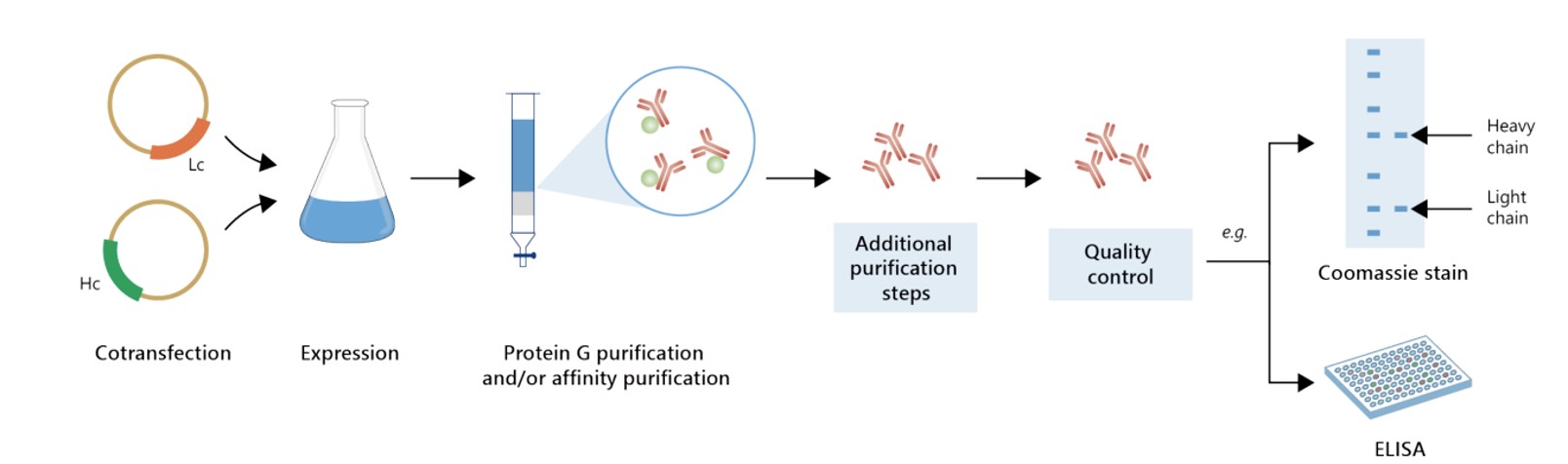
Fig.6 Milligram-scale recombinant antibody production.
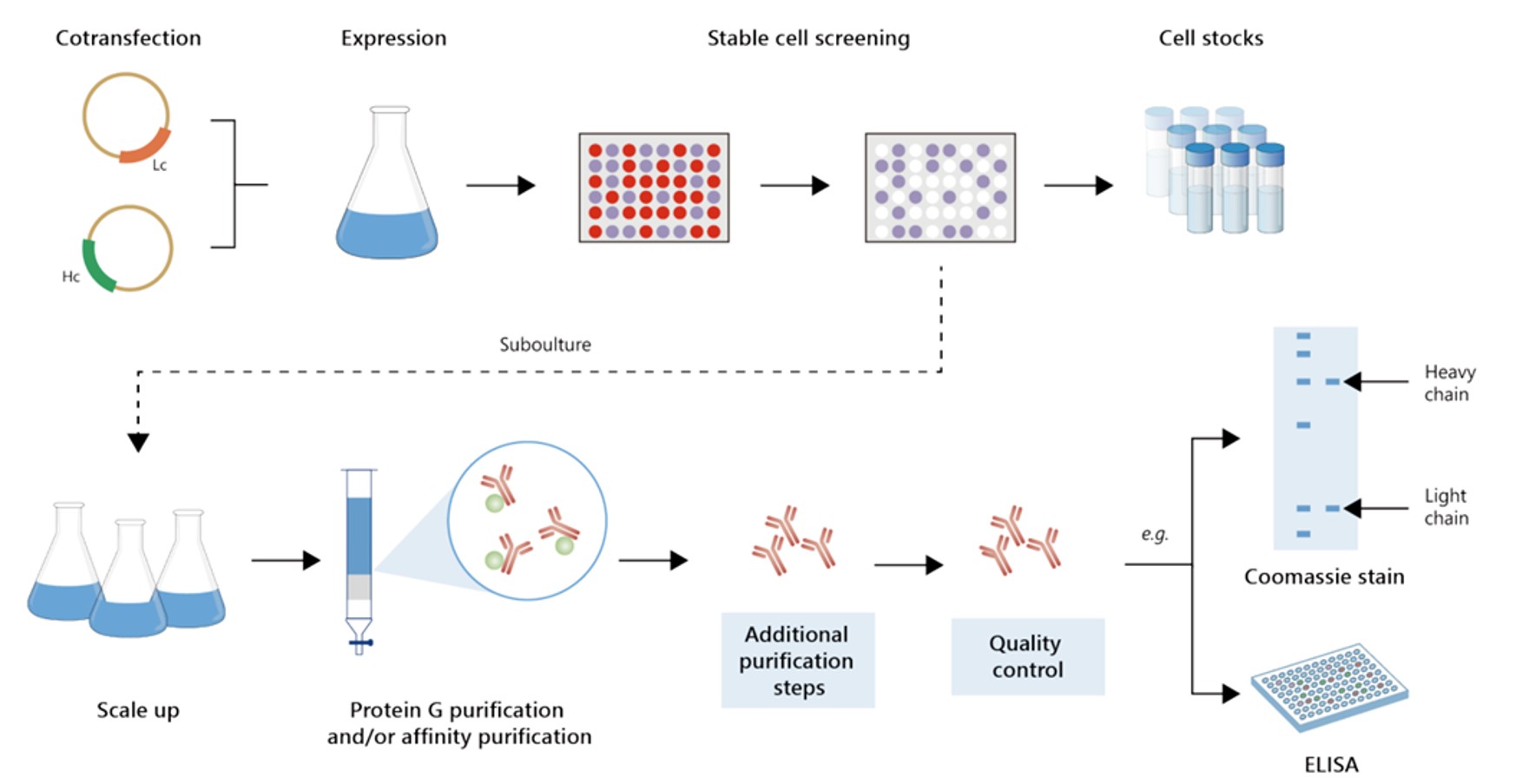 Fig.7 Gram-scale recombinant antibody production.
Fig.7 Gram-scale recombinant antibody production.
rAb Modalities
Creative Biolabs is committed to providing researchers with a wide range of cutting-edge, high-quality recombinant antibodies in multiple formats. From full-length antibodies to scFv, Fab, scFab, and Fc fusion proteins, we offer flexible solutions to accommodate various experimental requirements. Our goal is to support your research by delivering the best tools for accurate and reliable results.
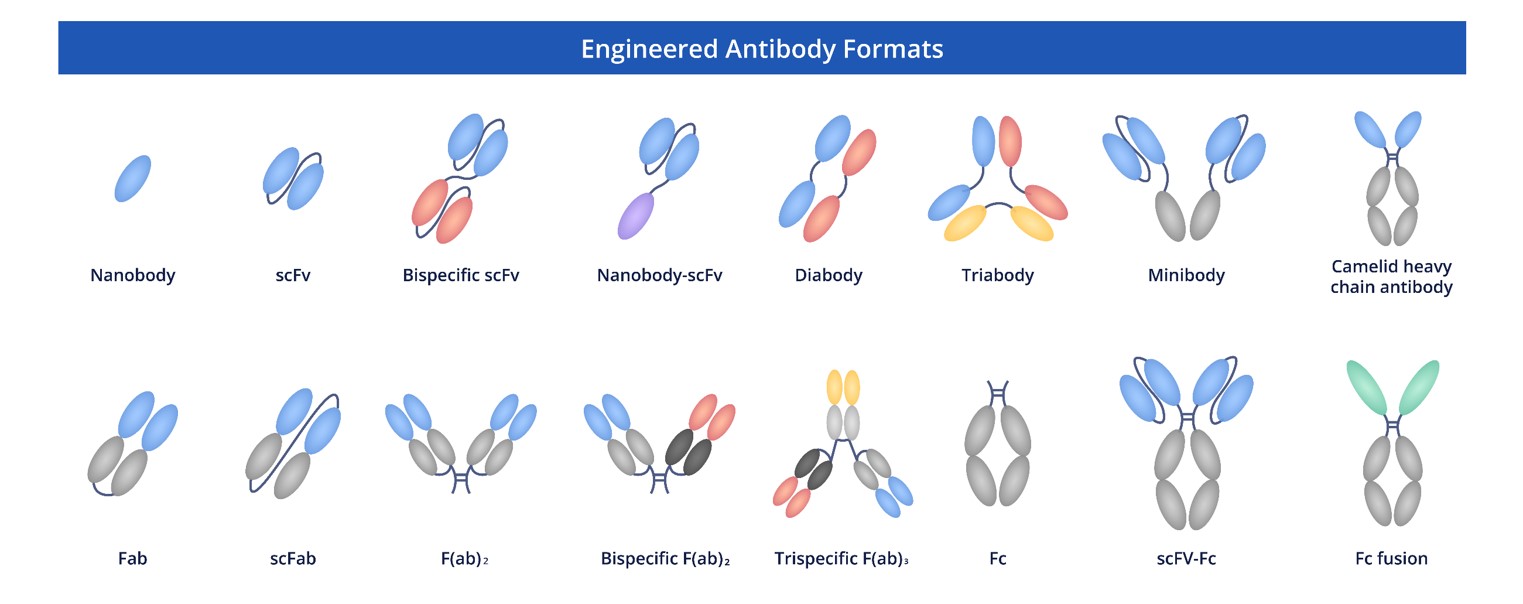 Fig.8 Full Length Anti-NT5E Recombinant Antibody Production and Modalities.
Fig.8 Full Length Anti-NT5E Recombinant Antibody Production and Modalities.
Drug Information Targeting NT5E
Table 1. Therapeutic approaches targeting NT5E in clinical development.
| Research phase | Company | Classification | Indications | Details |
| Launched - 2020 | Simcere Pharmaceutical |
Fixed-Dose Combinations Natural Products |
Amyotrophic lateral sclerosis | The product was approved and launched in China in 2020. In 2020, the combination was granted orphan drug designation in the U.S. for the treatment of amyotrophic lateral sclerosis. |
| Launched - 2001 | Daiichi Sankyo | Small Molecules | Amyotrophic lateral sclerosis | This is an antioxidant agent and free radical scavenger launched in Japan in 2001 by Mitsubishi Pharma (now Mitsubishi Tanabe Pharma) for the improvement of neurological symptoms and the treatment of disability and interference with daily activities at the acute stage of cerebral infarction. The drug is marketed in Japan as injections and bags for intravenous infusion. In 2015, the product was registered in Japan for the treatment of amyotrophic lateral sclerosis (ALS). In 2017, edaravone was approved and launched in the U.S. for the treatment of adult patients diagnosed with ALS. In 2018, Mitsubishi initiated a Managed Access Program (Early Access Program) in Europe for the treatment of ALS. In 2022, an oral formulation of edaravone for the treatment of ALS was launched in the U.S. and Japan in 2022 and 2023, respectively. |
| Phase III | AstraZeneca | Human Monoclonal Antibody | Cancer; Triple negative breast cancer | It is a monoclonal antibody against CD73 developed by AstraZeneca and its subsidiary MedImmune. The antibody is being evaluated in combination with durvalumab in a phase III clinical trial in patients with locally advanced, unresectable non-small cell lung cancer (NSCLC). Previously, a phase I/II trial was completed for the treatment of metastatic pancreatic adenocarcinoma. A phase II trial had been conducted in metastatic castration resistant prostate cancer (mCRPC) in combination with imaradenant; however, in 2020 the indication was terminated due to efficacy reasons. |
| Phase II | Agenus | Humanized Monoclonal Antibody | Cancer | It is a bispecific antibody against the transforming growth factor beta (TGFbeta) and CD73 in phase II clinical development at Agenus as an intravenous immunotherapeutic for the treatment of colorectal cancer liver metastases. Earlier on, Gilead initiated a phase I clinical trial of the product in patients with advanced/solid tumors; however, in 2021 this trial was prematurely terminated based on its clinical, pharmacokinetic and pharmacodynamic findings, with no safety concerns observed for the product. |
| Phase II | I-Mab Biopharma | Humanized Monoclonal Antibody | Cancer; Triple negative breast cancer | This is a humanized monoclonal antibody against ecto-5'-nucleotidase (CD73) in early clinical development at I-Mab Biopharma for the treatment of advanced solid tumors, both as monotherapy and in combination with other therapies. The company is conducting phase II clinical trial evaluating uliledlimab in combination with atezolizumab for the treatment of ovarian cancer as well as other selected solid tumors, including head and neck squamous cell carcinoma (HNSCC), non-small cell lung cancer (NSCLC), gastrointestinal cancer, and triple-negative breast cancer (TNBC). Phase I/II development is also evaluating the candidate in combination with toripalimab for the treatment of solid tumors. A combination with atezolizumab is also being evaluated by TRACON Pharmaceuticals in patients suffering from advanced or metastatic cancers. |
| Phase II | Arcus Biosciences | Small Molecules | Cancer | It is a small-molecule inhibitor of the CD73 immune checkpoint in phase II clinical development at Arcus Biosciences for the treatment of advanced or metastatic upper gastrointestinal tract adenocarcinomas and for metastatic non-small cell lung cancer (NSCLC). Early-stage trials are ongoing for metastatic castration-resistant prostate cancer (mCRPC), metastatic colorectal cancer, and pancreatic adenocarcinoma. |
| Phase I/II | Akeso Biopharma | Humanized Monoclonal Antibody | Cancer | This is a humanized monoclonal antibody targeting CD73 in early clinical development at Akeso Biopharma for the treatment of patients with refractory or relapsed advanced solid tumors, including EGFR-mutant locally advanced or metastatic non-squamous non-small cell lung cancer. A phase I trial has been completed that evaluated the candidate as a treatment for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19). |
| Phase I | Angel Pharmaceuticals | Humanized Monoclonal Antibody | Cancer | It is a humanized anti-CD73 antibody in early clinical development at Corvus Pharmaceuticals and Angel Pharmaceuticals for the treatment of adult patients with select advanced cancers, including HPV+ head and neck cancer, as monotherapy and in combination with ciforadenant or with pembrolizumab. A phase III trial had been ongoing for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19); however, the program was discontinued in 2021 due to strategic reasons. |
If you would like more information about our anti-NT5E recombinant antibodies, please feel free to contact us. Our team is ready to assist with any inquiries and is eager to collaborate with you, helping to advance your research and contribute to meaningful scientific discoveries.

