 Loading...
Loading...

SYK
Anti-SYK Recombinant Antibody Products
- Recombinant Anti-human SYK Antibody (MOB-937)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: IgG
- Application: WB, ELISA, IHC, FuncS
-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG
- Application: ELISA, WB, Dot, FuncS
- Mouse Anti-SYK Recombinant Antibody (clone 8C1A3) (VS3-XY1480)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, WB
- Rabbit Anti-SYK Polyclonal Antibody (VS3-WK1561)
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ELISA
-
- Species Reactivity: Human, Mouse, Rat
- Type: Rabbit IgG
- Application: WB, ELISA
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: WB
- Rabbit Anti-SYK Recombinant Antibody (HPAB-0225-YC) (HPAB-0225-YC)
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: IHC, FuncS
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: WB, ELISA
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, ELISA
- Rabbit Anti-SYK Recombinant Antibody; scFv Fragment (HPAB-0225-YC-S(P)) (HPAB-0225-YC-S(P))
-
- Species Reactivity: Human
- Type: Rabbit scFv
- Application: IHC, FuncS
- Rabbit Anti-SYK Recombinant Antibody; Fab Fragment (HPAB-0225-YC-F(E)) (HPAB-0225-YC-F(E))
-
- Species Reactivity: Human
- Type: Rabbit Fab
- Application: IHC, FuncS
- Recombinant Human Anti-human SYK Antibody Fab Fragment (MHH-937-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Fab
- Application: FC, ELISA, FuncS
- Recombinant Human Anti-human SYK Antibody scFv Fragment (MHH-937-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: scFv
- Application: ELISA, WB, IP, FuncS
- Recombinant Anti-human SYK Antibody Fab Fragment (MOB-937-F(E))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Fab
- Application: IF, FACS, FuncS
- Recombinant Anti-human SYK Antibody scFv Fragment (MOB-937-S(P))
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: scFv
- Application: ELISA, WB, FC, FuncS
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Cancer-related genes, Enzymes, FDA approved drug targets, Metabolic proteins
Intracellular
Cell type enhanced (Microglial cells, Kupffer cells, Macrophages, monocytes, B-cells, Plasma cells, Hofbauer cells)
Group enriched (neutrophil, myeloid DC, eosinophil, classical monocyte, memory B-cell, intermediate monocyte, plasmacytoid DC, non-classical monocyte, naive B-cell, NK-cell)
Cell line enhanced (Daudi, HEL, HL-60, HMC-1, REH, THP-1, U-698, U-937)
Interacts with LYN; phosphorylates SYK (By similarity). Interacts with RHOH (phosphorylated); regulates mast cells activation (By similarity). Interacts with NFAM1 (phosphorylated); probably involved in BCR signaling (By similarity). Interacts with VAV1 (via SH2 domain); phosphorylates VAV1 upon BCR activation. Interacts with GAB2 (phosphorylated); probably involved in IgE Fc receptor signaling (By similarity). Interacts (via its SH2 domains) with CD79A (via its phosphorylated ITAM domain); the interaction stimulates SYK autophosphorylation and activation (By similarity). Interacts with FCRL3 (PubMed:19843936, 11162587). Interacts (via SH2 domains) with FCER1G (via ITAM domain); activates SYK and mediates neutrophils and macrophages integrin-mediated activation (By similarity). Interaction with FCER1G in basophils triggers IL3-induced IL4 production (By similarity). Interacts with ITGB2 and FGR; involved in ITGB2 downstream signaling (By similarity). Interacts with ITGB3; upon activation by ITGB3 promotes platelet adhesion. Interacts (via SH2 domains) with TYROBP (via ITAM domain); involved in neutrophils and macrophages integrin-mediated activation (By similarity). Interacts with MSN and SELPLG; mediates the selectin-dependent activation of SYK by SELPLG. Interacts with BLNK (via SH2 domain). Interacts (via the second SH2 domain) with USP25 (via C-terminus); phosphorylates USP25 and regulates USP25 intracellular levels. Interacts (via SH2 domains) with CLEC1B (dimer). Interacts with CLEC7A; participates in leukocyte activation in presence of fungal pathogens. Interacts (phosphorylated) with SLA; may regulate SYK through CBL recruitment. Interacts with YWHAG; attenuates BCR-induced membrane translocation and activation of SYK. Interacts (via SH2 domains) with GCSAM; the interaction increases after B-cell receptor stimulation, resulting in enhanced SYK autophosphorylation and activity. Interacts with TNS2; leading to the phosphorylation of SYK (PubMed:22019427). Interacts with FLNA (via filamin repeat 5); docks SYK to the plasma membrane (PubMed:20713593). Interacts with CEACAM1; lipopolysaccharide activated neutrophils induce phosphorylation of SYK resulting in the formation of a complex including TLR4 and the phosphorylated form of SYK and CEACAM1, which in turn, recruits PTPN6 that dephosphorylates SYK, reducing the production of reactive oxygen species (ROS) and lysosome disruption, leading to a reduction of the inflammasome activity (By similarity). Interacts (via SH2 domains) with CEACAM20 (phosphorylated form); the interaction further enhances CEACAM20 phosphorylation (By similarity). Interacts with IL15RA (PubMed:15123770). (Microbial infection) Interacts with Epstein-Barr virus LMP2A.
Kinase, Transferase, Tyrosine-protein kinase

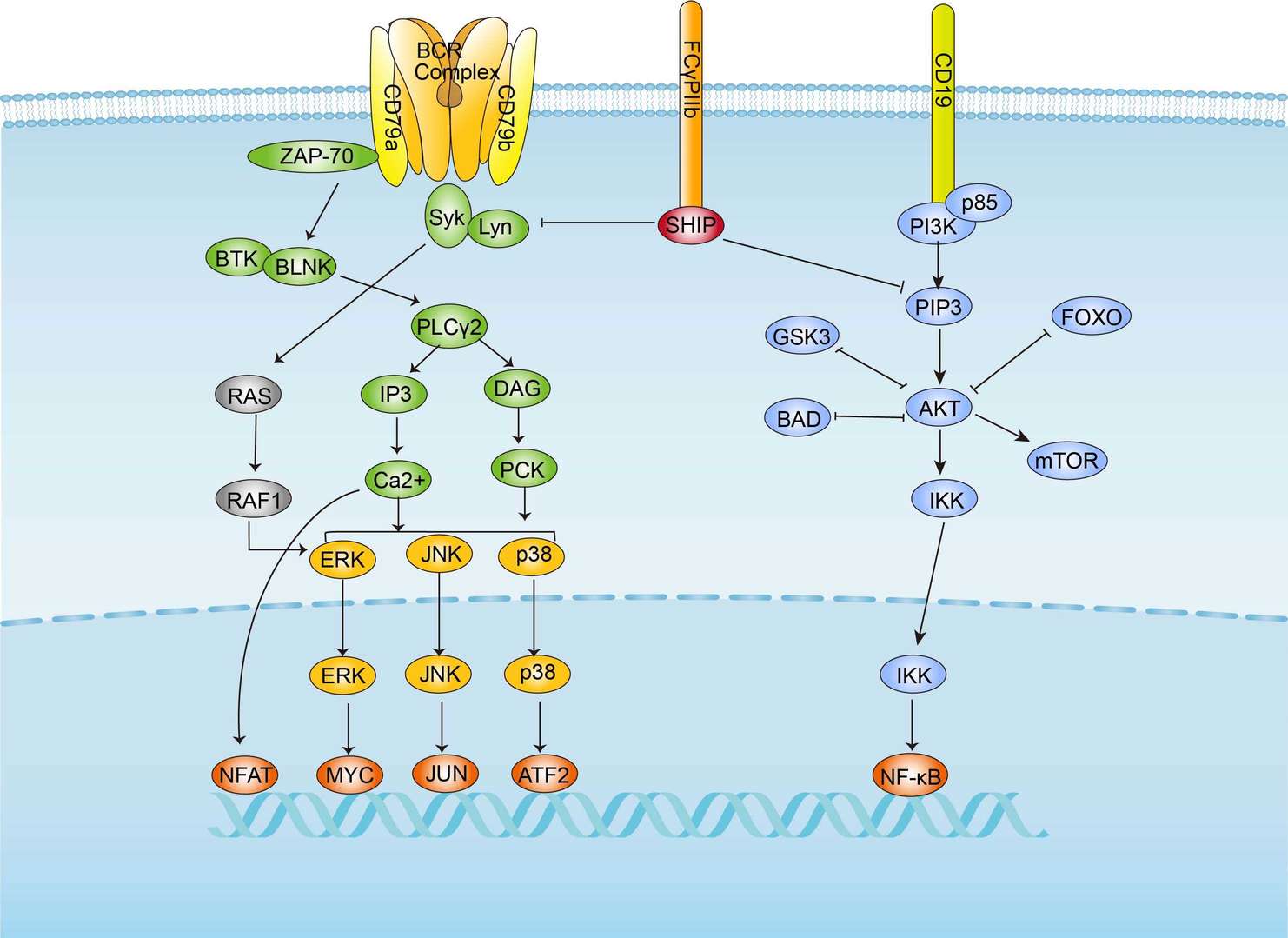 BCR Signaling Pathway
BCR Signaling Pathway
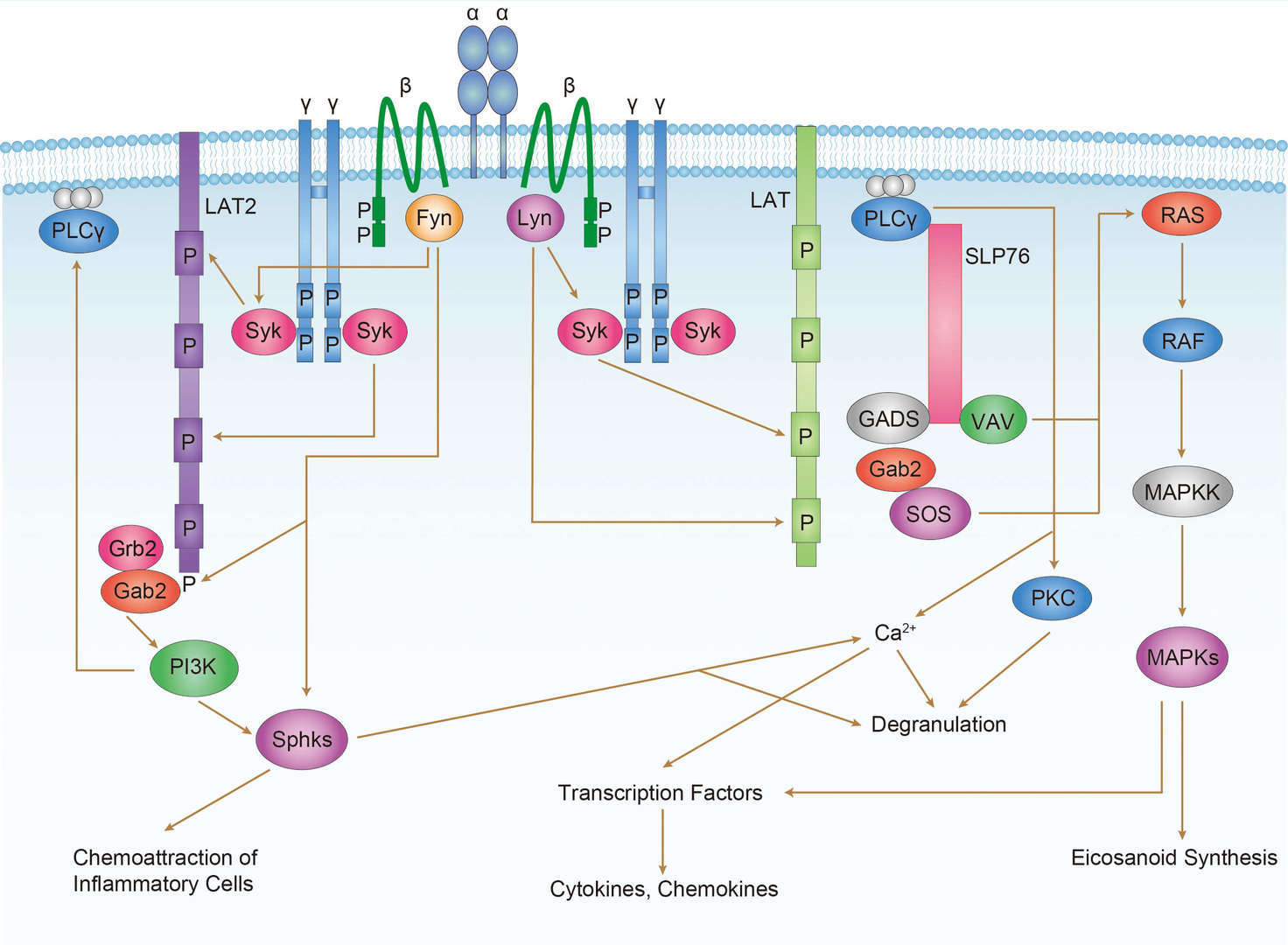 FcεR1 Signaling Pathway
FcεR1 Signaling Pathway