Afuco™ Anti-Human IL2 ADCC Recombinant Antibody (MT204), ADCC Enhanced
CAT#: AFC-395CL
Anti-IL2 ADCC Enhanced Antibody (MT204) is an ADCC enhanced antibody produced by our Afuco™ platform. MT204 is a humanized IgG1 antibody specific for interleukin-2 (IL-2) of human and rhesus monkey origin. MT204 has the potential to treat a wide variety of acute and chronic inflammatory diseases, including rheumatoid arthritis, asthma, acute transplant rejection, uveitis, psoriasis and multiple sclerosis. MT204 is the first humanized antibody targeting soluble human and non-human primate IL-2 by a unique mode of action, and has been shown in preclinical models to have inhibitory properties.




Specifications
- Host Species
- Humanized
- Derivation
- Humanized
- Type
- ADCC enhanced antibody
- Antibody Isotype
- IgG1
- Species Reactivity
- Human
- Related Disease
- Multiple Sclerosis; Rheumatoid Arthritis
Product Property
- Purity
- >95% as determined by Analysis by RP-HPLC & analysis by SDS-PAGE
- Storage
- ≥1 year at -20°C. If the reconstituted antibody cannot be used within two weeks, it should be aliquoted into smaller vials and stored at -20°C
Target
Customer Review
There are currently no Customer reviews or questions for AFC-395CL. Click the button above to contact us or submit your feedback about this product.
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
See other products for "Il2"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1081z | Mouse Anti-IL2 Recombinant Antibody (clone 23B4) | WB, ELISA, FC, FuncS | Mouse IgG1, κ |
| PABL-240 | Mouse Anti-IL2 Recombinant Antibody (clone LNKB-2) | WB, IHC, FuncS | Mouse IgG |
| PABL-609 | Mouse Anti-Il2 Recombinant Antibody (PABL-609) | FC, FuncS, IHC, ELISA | Mouse IgG |
| PABL-610 | Mouse Anti-Il2 Recombinant Antibody (clone S4B6) | ELISA, FC, FuncS | Mouse IgG |
| MOB-0241CT | Recombinant Mouse anti-Human IL2 Monoclonal antibody (25-0) | ELISA |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NABG-093 | Recombinant Anti-Mouse Il2 VHH Single Domain Antibody | ELISA, IHC, FC, FuncS | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-412CL | Human Anti-IL2 Recombinant Antibody (TAB-412CL) | FuncS | Human IgG1 |
| TAB-556LC | Human Anti-IL2 Recombinant Antibody (TAB-556LC) | ELISA, FC | Humanized antibody |
| TAB-556LC-S(P) | Human Anti-IL2 Recombinant Antibody; scFv Fragment (TAB-556LC-S(P)) | ELISA, FC | Humanized scFv |
| TAB-556LC-F(E) | Human Anti-IL2 Recombinant Antibody; Fab Fragment (TAB-556LC-F(E)) | ELISA, FC | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-240 | Recombinant Mouse Anti-IL2 Antibody scFv Fragment (LNKB-2) | WB, IHC, FuncS | scFv |
| PSBL-604 | Mouse Anti-Il2 Recombinant Antibody (clone JES6-1); scFv Fragment | Neut | Mouse scFv |
| PSBL-605 | Mouse Anti-Il2 Recombinant Antibody (clone S4B6); scFv Fragment | WB, ELISA, FuncS | Mouse scFv |
| HPAB-0112-YC-S(P) | Human Anti-IL2 Recombinant Antibody; scFv Fragment (HPAB-0112-YC-S(P)) | ELISA, FC, FuncS | Human scFv |
| FAMAB-0179-YC-S(P) | Human Anti-IL2 Recombinant Antibody (clone C5); scFv Fragment | ELISA, WB | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-240 | Mouse Anti-IL2 Recombinant Antibody (clone LNKB-2); Fab Fragment | WB, IHC, FuncS | Mouse Fab |
| PFBL-604 | Mouse Anti-Il2 Recombinant Antibody (clone JES6-1); Fab Fragment | Neut | Mouse Fab |
| PFBL-605 | Mouse Anti-Il2 Recombinant Antibody (clone S4B6); Fab Fragment | WB, ELISA, FuncS | Mouse Fab |
| HPAB-0112-YC-F(E) | Human Anti-IL2 Recombinant Antibody; Fab Fragment (HPAB-0112-YC-F(E)) | ELISA, FC, FuncS | Human Fab |
| FAMAB-0179-YC-F(E) | Human Anti-IL2 Recombinant Antibody (clone C5); Fab Fragment | ELISA, WB | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-557LC | Human Anti-IL2 Recombinant Antibody (TAB-557LC) | ELISA, FC, Inhib, FuncS | Human IgG1 |
| TAB-558LC | Human Anti-IL2 Recombinant Antibody (TAB-558LC) | ELISA, FC, FuncS | Human IgG1 |
| TAB-557LC-S(P) | Human Anti-IL2 Recombinant Antibody; scFv Fragment (TAB-557LC-S(P)) | ELISA, FC | Human scFv |
| TAB-558LC-S(P) | Human Anti-IL2 Recombinant Antibody; scFv Fragment (TAB-558LC-S(P)) | ELISA, FC | Human scFv |
| TAB-557LC-F(E) | Human Anti-IL2 Recombinant Antibody; Fab Fragment (TAB-557LC-F(E)) | ELISA, FC | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-559LC | Mouse Anti-IL2 Recombinant Antibody (TAB-559LC) | ELISA, FC, FuncS | Mouse IgG |
| TAB-559LC-S(P) | Mouse Anti-IL2 Recombinant Antibody; scFv Fragment (TAB-559LC-S(P)) | ELISA, FC | Mouse scFv |
| TAB-559LC-F(E) | Mouse Anti-IL2 Recombinant Antibody; Fab Fragment (TAB-559LC-F(E)) | ELISA, FC | Mouse Fab |
| PABX-123 | Recombinant Mouse Anti-Interleukin 2 Antibody | WB, ELISA, Neut, FuncS | IgG |
| PABX-123-F (E) | Recombinant Mouse Anti-Interleukin 2 Antibody Fab Fragment | WB, ELISA, Neut, FuncS | Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-182LC | Recombinant Anti-Human IL2 Antibody (Non-glycosylated) | ELISA | Human antibody |
| Gly-183LC | Recombinant Anti-Human IL2 Antibody (Non-glycosylated) | ELISA | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| BRD-0283MZ | Chicken Anti-Interleukin-2 Polyclonal IgY | WB, Indirect ELISA | Chicken antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1306CQ | Rat Anti-IL2 Recombinant Antibody (clone 17H12) | Neut | Rat IgG2a |
| NEUT-1307CQ | Mouse Anti-IL2 Recombinant Antibody (clone 297C16F11) | IHC, Neut | Mouse IgG2a, κ |
| NEUT-1308CQ | Mouse Anti-IL2 Recombinant Antibody (VMC194) | FuncS, Neut | Mouse IgG2a, κ |
| NEUT-1310CQ | Mouse Anti-IL2 Recombinant Antibody (clone CBL121) | Neut, WB, IHC | Mouse IgG1 |
| NEUT-1311CQ | Mouse Anti-IL2 Recombinant Antibody (clone CBL211) | DB, IHC, Neut | Mouse IgG2a, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1309CQ | Mouse Anti-IL2 Recombinant Antibody (VMC195) | FC, Block | Mouse IgG1 |
| NEUT-1315CQ | Rat Anti-Il2 Recombinant Antibody (clone 1A12), Biotin | Block, ELISA, WB | Rat IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1801 | Rabbit Anti-IL2 Recombinant Antibody (clone DS1801AB) | ICC, IHC-P, WB | Rabbit IgG |
| MOR-4612 | Rabbit Anti-IL2 Recombinant Antibody (clone TH125DS) | WB, IHC-P | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1024-XY277 | Mouse Anti-NHP IL2 Recombinant Antibody (clone 5355) | IF, ELISA | Mouse IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY132 | CytoStream™ Rat Anti-IL2 Recombinant Antibody (clone MQ1-17H12) | FC | Rat IgG2a, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-FY107 | Mouse Anti-IL2 scFv-Fc Chimera (VS-0325-FY107) | FC, IHC, ELISA, Block | Mouse IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0625-YC175 | Recombinant Anti-IL2 Eliminating Antibody, pH-Sensitive (VS-0625-YC175) | Antigen-Sweeping In Vivo. | Human IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC184 | SmartAb™ Recombinant Anti-IL2 pH-dependent Antibody (VS-0825-YC184) | WB, ELISA, FC, IHC | Human IgG1 |
Popular Products

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: Neut, ELISA, IF, IP, FuncS, FC, ICC

Application: IF, IP, Neut, FuncS, ELISA, FC, ICC

Application: ELISA, IP, FC, FuncS, Neut, IF, ICC

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC

Application: WB, Neut, ELISA, IF, IP, FuncS, FC

Application: IHC, ELISA, FC, WB, ADCC, FuncS
-2.png)
Application: WB, ELISA, FC, IHC, IP

Application: SPR, Inhib, FuncS

Application: FC, FRET, Internalization
-3.jpg)
Application: Neut

Application: ELISA, WB

Application: ELISA, FC, FuncS
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.







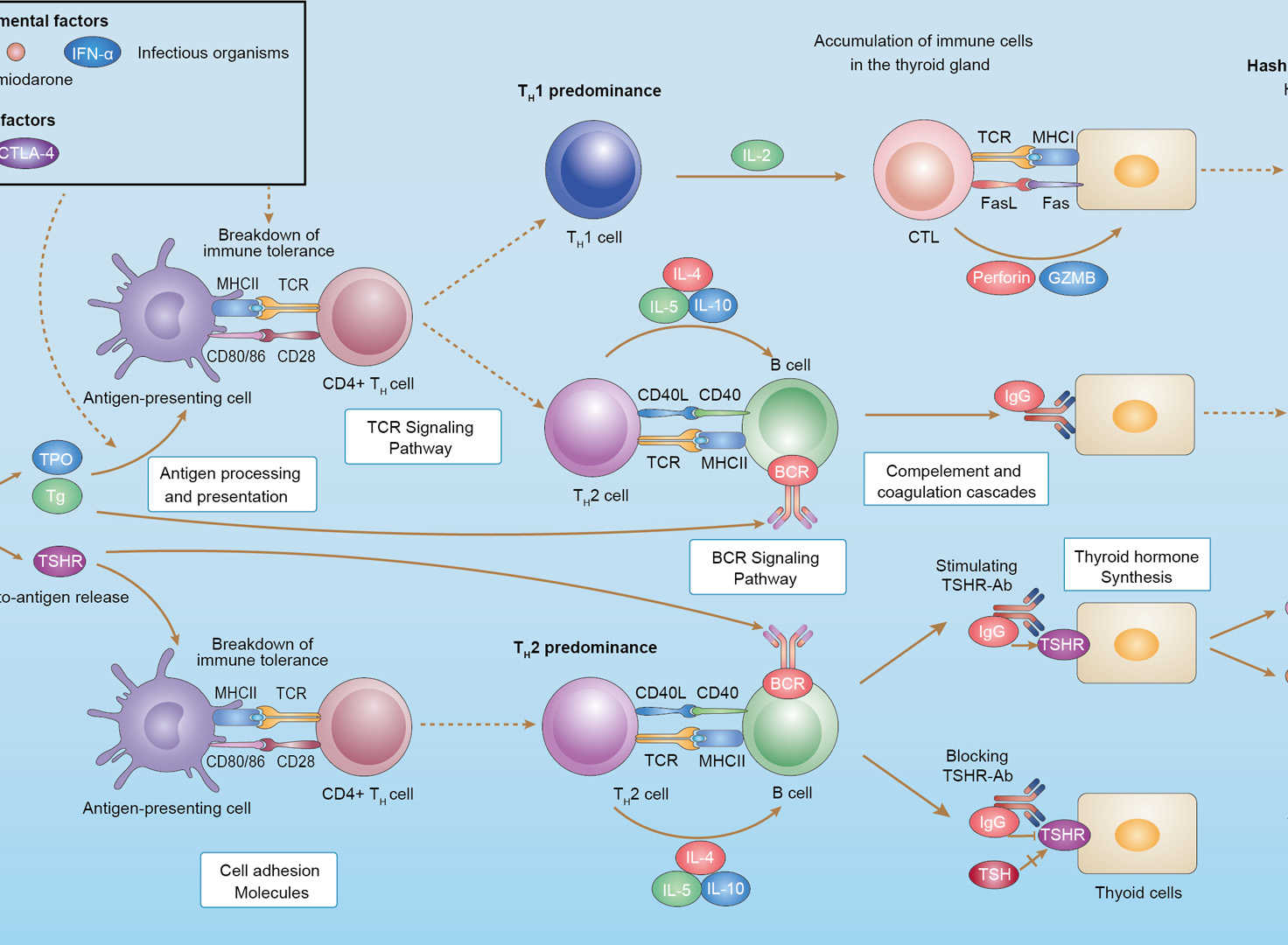 Autoimmune Thyroid Disease
Autoimmune Thyroid Disease
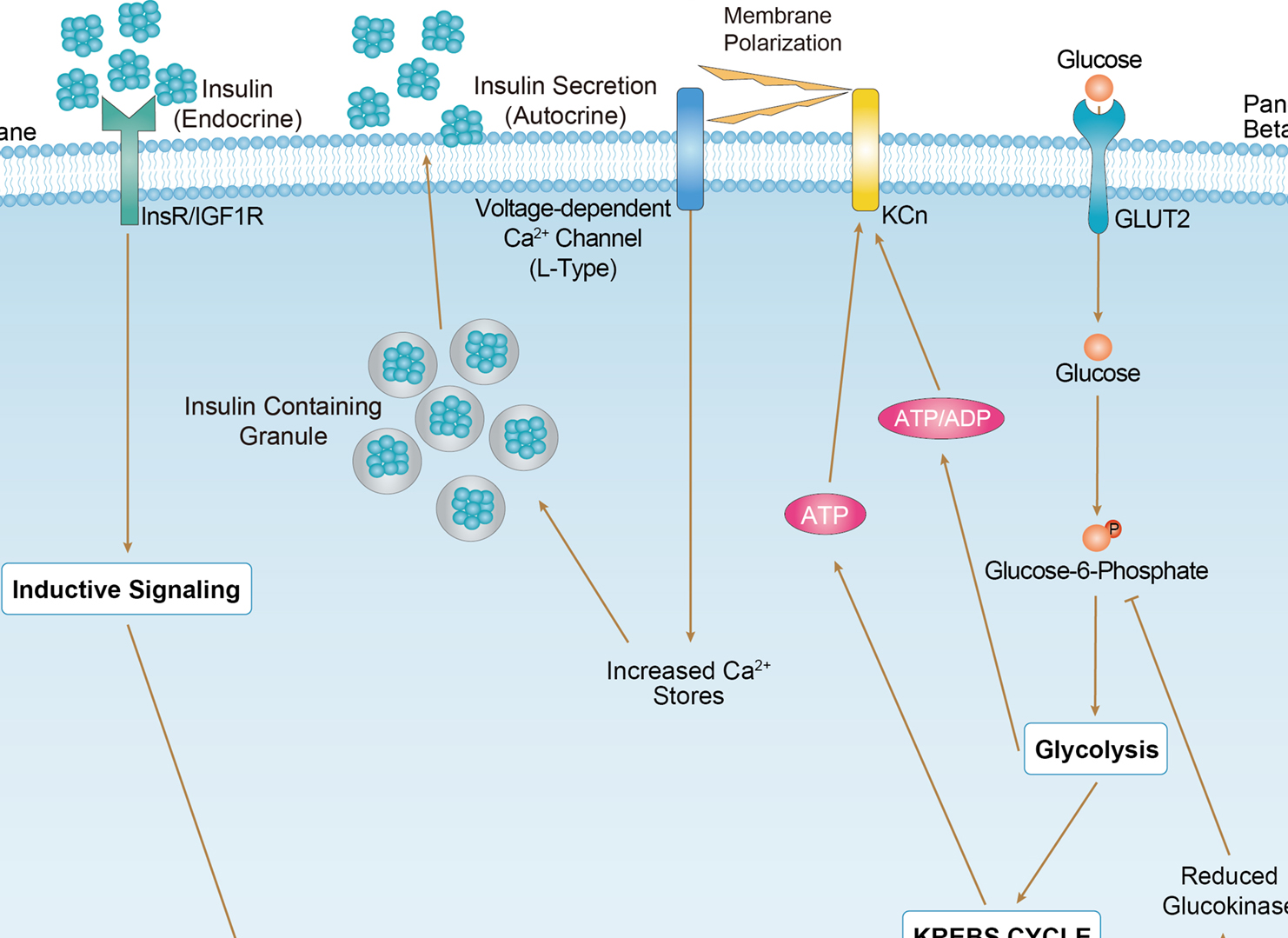 Maturity Onset Diabetes of the Young
Maturity Onset Diabetes of the Young










