Anti-CD19 Recombinant Antibody Products
 Loading...
Loading...Anti-CD19 Products
 Loading...
Loading...- anti-CD19 immunotoxin HD37 (scFv)-PE (AGTO-L047E)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Functional assay
- Anti-Human CD19 Recombinant Antibody (TAB-108CL) (TAB-108CL)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Antibody
- Application: FC, ADCC, FuncS
- Mouse Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1611CL-F(E)) (TAB-1611CL-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: Depletion, FuncS
- Recombinant Humanized Anti-CD19 Antibody (HPAB-0036LY) (HPAB-0036LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Application: WB, IHC
- Anti-Human CD19 Recombinant Antibody (TAB-431CQ)
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: IgG1, κ
- Application: ELISA, IHC, FC, IP, IF, FuncS
- Human Anti-CD19 Recombinant Antibody (HPAB-0268-CN) (HPAB-0268-CN)
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human IgG1
- Application: ELISA, FC
- Human Anti-CD19 Recombinant Antibody (HPAB-0418LY) (HPAB-0418LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: Cyt, BLI, FC, FuncS, ELISA, WB, RIA
- Human Anti-CD19 Recombinant Antibody (HPAB-0419LY) (HPAB-0419LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: ELISA, WB, FC, RIA
- Mouse Anti-CD19 Recombinant Antibody (TAB-1611CL) (TAB-1611CL)
-
- Species Reactivity: Human
- Type: Mouse IgG2a, κ
- Application: Depletion, FuncS
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: WB, FC, ICC, IF, FuncS
- Anti-Human CD19 Recombinant Antibody (396) (TAB-1625CL)
-
- Derivation: Humanized
- Type: Humanized antibody
- Application: Depletion, ELISA
- Human Anti-CD19 Recombinant Antibody (TAB-1626CL) (TAB-1626CL)
-
- Type: Fully human antibody
- Application: Depletion
- Human Anti-CD19 Recombinant Antibody (TAB-1627CL) (TAB-1627CL)
-
- Type: Fully human antibody
- Application: Depletion
- Human Anti-CD19 Recombinant Antibody (TAB-1612CL) (TAB-1612CL)
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized IgG1
- Application: Depletion, FuncS
- Human Anti-CD19 Recombinant Antibody (TAB-1613CL) (TAB-1613CL)
-
- Derivation: Chimeric (mouse/human)
- Species Reactivity: Human
- Type: Chimeric (mouse/human) IgG1
- Application: Cyt, FC, FuncS, Inhib
- Human Anti-CD19 Recombinant Antibody (TAB-1614CL) (TAB-1614CL)
-
- Derivation: Chimeric (mouse/human)
- Species Reactivity: Human
- Type: Chimeric (mouse/human) IgG
- Application: FuncS
- Human Anti-CD19 Recombinant Antibody (TAB-1616CL) (TAB-1616CL)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody (TAB-1617CL) (TAB-1617CL)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody (TAB-1618CL) (TAB-1618CL)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody (TAB-1619CL) (TAB-1619CL)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, WB
-
- Derivation: Human
- Species Reactivity: Human
- Type: ADCC enhanced antibody
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized IgG1, κ
- Application: ELISA, FC, IP, FuncS, IF, Neut, ICC
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: IgG1 - kappa
- Application: IF, IP, Neut, FuncS, ELISA, FC, WB
- Mouse Anti-CD19 Recombinant Antibody (clone PGC3D6H10) (HPAB-0696LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA, WB, FC, IF
- Human Anti-CD19 Recombinant Antibody Fab Fragment (TAB-1626CL-F(E)) (TAB-1626CL-F(E))
-
- Type: Fully human antibody
- Application: Depletion
- Human Anti-CD19 Recombinant Antibody Fab Fragment (TAB-1627CL-F(E)) (TAB-1627CL-F(E))
-
- Type: Fully human antibody
- Application: Depletion
- Mouse Anti-CD19 Recombinant Antibody (clone ME-CD20) (MOB-0278MZ)
-
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: IHC
- Human Anti-CD19 Recombinant Antibody (HPAB-0264-CN) (HPAB-0264-CN)
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized IgG1, κ
- Application: ELISA, FC
- Human Anti-CD19 Recombinant Antibody (HPAB-0265-CN) (HPAB-0265-CN)
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized IgG1, κ
- Application: ELISA, FC
- Human Anti-CD19 Recombinant Antibody (HPAB-0266-CN) (HPAB-0266-CN)
-
- Derivation: Humanized
- Species Reactivity: Human, Cynomolgus monkey
- Type: Humanized IgG
- Application: ELISA, FC
- Human Anti-CD19 Recombinant Antibody (HPAB-0267-CN) (HPAB-0267-CN)
-
- Derivation: Humanized
- Species Reactivity: Human, Cynomolgus monkey
- Type: Humanized IgG
- Application: ELISA, FC
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1612CL-F(E)) (TAB-1612CL-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: Depletion, FuncS
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1613CL-F(E)) (TAB-1613CL-F(E))
-
- Derivation: Chimeric (mouse/human)
- Species Reactivity: Human
- Type: Chimeric (mouse/human) Fab
- Application: Depletion, FuncS
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1614CL-F(E)) (TAB-1614CL-F(E))
-
- Derivation: Chimeric (mouse/human)
- Species Reactivity: Human
- Type: Chimeric (mouse/human) Fab
- Application: FuncS
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1616CL-F(E)) (TAB-1616CL-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1617CL-F(E)) (TAB-1617CL-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1618CL-F(E)) (TAB-1618CL-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1619CL-F(E)) (TAB-1619CL-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1612CL-S(P)) (TAB-1612CL-S(P))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized scFv
- Application: Depletion, FuncS
- Mouse Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1613CL-S(P)) (TAB-1613CL-S(P))
-
- Derivation: Chimeric (mouse/human)
- Species Reactivity: Human
- Type: Mouse scFv
- Application: Depletion, FuncS
- Mouse Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1614CL-S(P)) (TAB-1614CL-S(P))
-
- Derivation: Chimeric (mouse/human)
- Species Reactivity: Human
- Type: Mouse scFv
- Application: FuncS
- Human Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1616CL-S(P)) (TAB-1616CL-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1617CL-S(P)) (TAB-1617CL-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, WB
- Human Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1618CL-S(P)) (TAB-1618CL-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, WB
- Anti-Human CD19 Recombinant Antibody (cHD37) (TAB-1621CL)
-
- Derivation: Chimeric (mouse/human)
- Type: Chimeric antibody (mouse/human)
- Application: ADCC, FuncS
- Anti-Human CD19 Recombinant Antibody Fab Fragment (cHD37) (TAB-1621CL-F(E))
-
- Derivation: Chimeric (mouse/human)
- Type: Chimeric antibody (mouse/human)
- Application: FuncS
- Human Anti-CD19 Recombinant Antibody (HPAB-0420LY) (HPAB-0420LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: ELISA, WB, FC, RIA
- Human Anti-CD19 Recombinant Antibody (HPAB-0421LY) (HPAB-0421LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: ELISA, WB, FC, RIA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-0269-CN) (HPAB-0269-CN)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA, FC
- Human Anti-CD19 Recombinant Antibody (HPAB-0117LY) (HPAB-0117LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Humanized IgG1
- Application: FC
- Humanized Anti-CD19 Recombinant Antibody (HPAB-0023LY) (HPAB-0023LY)
-
- Species Reactivity: Human
- Type: Humanized IgG1
- Application: WB, FC, Cyt
- Human Anti-CD19 Recombinant Antibody (HPAB-616-FY) (HPAB-616-FY)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA
- Human Anti-CD19 Recombinant Antibody (clone 2A3) (HPAB-M0025-YC)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3158LY) (HPAB-3158LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3159LY) (HPAB-3159LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3160LY) (HPAB-3160LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3161LY) (HPAB-3161LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3162LY) (HPAB-3162LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3163LY) (HPAB-3163LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3164LY) (HPAB-3164LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (HPAB-3165LY) (HPAB-3165LY)
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody; Fab Fragment (HPAB-0305-FY-F(E)) (HPAB-0305-FY-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA
- Mouse Anti-CD19 Recombinant Antibody (TAB-1622CL) (TAB-1622CL)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA, FC, Apop, ADCC, FuncS
- Mouse Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1622CL-F(E)) (TAB-1622CL-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA, FC, Apop, ADCC, FuncS
- Mouse Anti-CD19 Recombinant Antibody; Fab Fragment (TAB-1623CL-F(E)) (TAB-1623CL-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA, FC
- Mouse Anti-CD19 Recombinant Antibody (TAB-1623CL) (TAB-1623CL)
-
- Species Reactivity: Human
- Type: Mouse IgG
- Application: ELISA, FC
- Anti-Human CD19 Recombinant Antibody (381) (TAB-1624CL)
-
- Application: FC, Inhib, ELISA
- Anti-Human CD19 Recombinant Antibody Fab Fragment (HD37) (TAB-1620CL-F(E))
-
- Application: FuncS
- Anti-CD19 immunotoxin HD37 (IgG)-PAP (AGTO-G076P)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Function study
- anti-CD19 immunotoxin HD37 (scFv)-Sap (AGTO-L047S)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Functional assay
- anti-CD19 immunotoxin HD37 (scFv)-PAP (AGTO-L047P)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Functional assay
- Anti-CD19 immunotoxin HD37 (IgG)-PE (AGTO-G076E)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Function study
- Anti-CD19 immunotoxin HD37 (IgG)-DT (AGTO-G076D)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Function study
- Anti-CD19 immunotoxin HD37 (IgG)-RTA (AGTO-G076R)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Function study
- anti-CD19 immunotoxin HD37 (scFv)-DT (AGTO-L047D)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Functional assay
- anti-CD19 immunotoxin HD37 (scFv)-RTA (AGTO-L047R)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Functional assay
- Anti-CD19 immunotoxin HD37 (IgG)-Sap (AGTO-G076S)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Function study
- Anti-Human CD19 Recombinant Antibody (HD37) (TAB-1620CL)
-
- Application: FuncS
- Mouse Anti-CD19 Recombinant Antibody; scFv Fragment (TAB-1611CL-S(P)) (TAB-1611CL-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: Depletion, FuncS
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1, κ
- Application: ELISA, IP, FC, FuncS, Neut, IF, ICC
- Anti-CD19 (huB4)-SPDB-DM4 ADC (ADC-020LCT)
-
- Target: CD19
- Linker: SPDB (N-succinimidyl 4-(2-pyridyldithio)butyrate)
- Drug: DM4 (N2'-Deacetyl-N2'-(4-mercapto-4-methyl-1-oxopentyl)maytansine)
- Anti-CD19 (hBU12)-mcMMAF ADC (ADC-017LCT)
-
- Target: CD19
- Linker: Mc (maleimidocaproyl)
- Drug: MMAF (Monomethyl auristatin F)
- Anti-CD19 (hBU12)-Mc-MMAE ADC (ADC-019LCT)
-
- Target: CD19
- Linker: Mc (maleimidocaproyl)
- Drug: MMAE (Monomethyl auristatin E)
- Anti-CD19 (clone huB4)-mc-MMAF ADC (ADC-045LZY)
-
- Target: CD19
- Linker: mc (maleimidocaproyl)
- Drug: MMAF (monomethyl auristatin F)
- Anti-CD19 (Denintuzumab)-Mc-MMAF ADC (ADC-175LCT)
-
- Target: CD19
- Linker: Mc (maleimidocaproyl)
- Drug: MMAF (Monomethyl auristatin F)
- Anti-CD19 (clone huB4)-SMCC-DM1 ADC (ADC-157LZY)
-
- Target: CD19
- Linker: SMCC (4-[N-maleimidomethyl] cyclohexane-1 carboxylhydrazide).
- Drug: DM1(N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine)
- Anti-CD19 (clone MDX1435)-vc-duocarmycin ADC (ADC-163LZY)
-
- Target: CD19
- Linker: VC (valine-citrulline)
- Drug: duocarmycin
-
- Target: CD19
- Linker: mc-vc (maleimidocaproyl-valine-alanine)
- Drug: pyrrolobenzodiazepine (PBD) dimers SGD-1882
- Anti-CD19 (clone hBU12)-vcPAB-MMAE ADC (ADC-150CL)
-
- Target: CD19
- Linker: VC-PAB (valine-citrulline-p-aminobenzoyloxycarbonyl)
- Drug: MMAE (Monomethyl auristatin E)
- Anti-CD19 (clone BU12)-MC-MMAF ADC (ADC-189CL)
-
- Target: CD19
- Linker: Mc (maleimidocaproyl)
- Drug: MMAF (Monomethyl auristatin F)
- Anti-CD19 (clone B496)-SPP-DM1 ADC (ADC-181LZY)
-
- Target: CD19
- Linker: SPP (N-succinimidyl-4-(2-pyridyldithio)pentanoate)
- Drug: DM1(N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine)
- Anti-CD19 (clone B496)-MCC-DM1 ADC (ADC-182LZY)
-
- Target: CD19
- Linker: MCC (Maleimidomethyl cyclohexane-1-carboxylate)
- Drug: DM1 (N2'-deacetyl-N2'-(3-mercapto-1-oxopropyl)-maytansine)
- Anti-CD19 (clone B496)-mc-vc-PABC-MMAE ADC (ADC-183LZY)
-
- Target: CD19
- Linker: mc-VC-PABC (maleimidocaproyl valine-citrulline-p-aminobenzyl carbamoyl)
- Drug: MMAE (monomethyl auristatin E)
- Anti-CD19 (clone B496)-mc-MMAF ADC (ADC-184LZY)
-
- Target: CD19
- Linker: mc (maleimidocaproyl)
- Drug: MMAF (monomethyl auristatin F)
- Anti-CD19 (hBU12)-Mc-MMAF ADC (SGN-19A) (ADC-L034)
-
- Target: CD19
- Linker: Mc (maleimidocaproyl)
- Drug: MMAF (Monomethyl auristatin F)
-
- Host Species: Human
- Specificity: Human
- Type: Humanized antibody
- Classification: Fc glycosylation/Non fucosylated
- Glycosylation site: Asn297 in Fc region
-
- Host Species: Human
- Specificity: Human
- Type: Chimeric antibody (mouse/human)
- Classification: Fc glycosylation/Low fucosylated
- Glycosylation site: Asn297 in Fc region
- Glycoform (ratio): GN-GN-M-(M-GN)2
-
- Host Species: Human
- Specificity: Human
- Type: Chimeric antibody (mouse/human)
- Classification: Fc glycosylation/Low fucosylated
- Glycosylation site: Asn297 in Fc region
- Glycoform (ratio): GN-GN-M-(M-GN)2
-
- Host Species: Mouse
- Specificity: Human
- Type: Mouse antibody
- Classification: Fc glycosylation
- Glycosylation site: Fc region
- Glycoform (ratio): G2-NANA
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Creative Biolabs offers cutting-edge recombinant antibodies targeting CD19, designed to support and advance biomedical research. Leveraging our advanced platforms, we ensure that each product adheres to the highest quality and performance standards. Our extensive portfolio of recombinant products is crafted to deliver exceptional value and reliability. In addition to our high-quality antibodies, we are committed to providing outstanding technical support, assisting researchers at every phase of their projects. Partnering with Creative Biolabs means gaining access to innovative solutions and a dedicated ally in your scientific journey.
CD19: A Key Target in B Cell Malignancies and Immunotherapy
CD19 is a cell surface protein predominantly expressed on B cells and plays a crucial role in B cell development and activation. It has become an important therapeutic target, particularly in B cell-related malignancies like chronic lymphocytic leukemia (CLL) and certain types of non-Hodgkin lymphoma (NHL). The targeting of CD19 is a promising strategy in immunotherapy, especially with CAR-T cell therapies, where genetically modified T cells are engineered to specifically recognize and kill CD19-positive B cells. CD19's restricted expression to B cells makes it an ideal target for selective treatment, minimizing off-target effects. Furthermore, the advent of monoclonal antibodies targeting CD19 has led to significant advances in treating diseases like CLL and diffuse large B-cell lymphoma. However, challenges remain, such as the development of resistance and the potential for targeting normal B cells, which may cause immune deficiencies. Despite these hurdles, CD19 continues to be a central focus in the development of innovative treatments for hematological malignancies.
Alternative Names
Complement CD19 Molecule; B-Lymphocyte Surface Antigen B4; T-Cell Surface Antigen Leu-12; Differentiation Antigen CD19; CD19 Antigen; B-Lymphocyte Antigen CD19; CVID3; B4
Background
This gene encodes a member of the immunoglobulin gene superfamily. Expression of this cell surface protein is restricted to B cell lymphocytes. This protein is a reliable marker for pre-B cells but its expression diminishes during terminal B cell differentiation in antibody secreting plasma cells. The protein has two N-terminal extracellular Ig-like domains separated by a non-Ig-like domain, a hydrophobic transmembrane domain, and a large C-terminal cytoplasmic domain. This protein forms a complex with several membrane proteins including complement receptor type 2 (CD21) and tetraspanin (CD81) and this complex reduces the threshold for antigen-initiated B cell activation. Activation of this B-cell antigen receptor complex activates the phosphatidylinositol 3-kinase signalling pathway and the subsequent release of intracellular stores of calcium ions. This protein is a target of chimeric antigen receptor (CAR) T-cells used in the treatment of lymphoblastic leukemia. Mutations in this gene are associated with the disease common variable immunodeficiency 3 (CVID3) which results in a failure of B-cell differentiation and impaired secretion of immunoglobulins. CVID3 is characterized by hypogammaglobulinemia, an inability to mount an antibody response to antigen, and recurrent bacterial infections.
Anti-CD19 rAb Products
We are dedicated to accelerating research and advancing scientific discoveries by providing premium anti-CD19 recombinant antibody products. Our offerings are not only of exceptional quality but also deliver outstanding value. In addition, we back our products with comprehensive technical support, ensuring researchers have the resources they need to succeed at every stage of their work. Through our commitment to excellence, we aim to be a trusted partner in driving forward cutting-edge research in immunology and related fields.
| Cat. No. | Product Name | Target Species | Host Species | Applications |
| TAB-108CL | Anti-Human CD19 Recombinant Antibody (TAB-108CL) | Human | Human | FC; ADCC; FuncS |
| TAB-431CQ | Anti-Human CD19 Recombinant Antibody | Human | Human | ELISA; IHC; IF; IP; FC; Funcs |
| HPAB-0418LY | Human Anti-CD19 Recombinant Antibody (HPAB-0418LY) | Human | Human | Cyt; BLI; FC; FuncS; ELISA; WB; RIA |
| HPAB-0988WJ | Human Anti-CD19 Recombinant Antibody (HPAB-0988WJ) | Human | Human | ELISA; FC |
| HPAB-0367-FY | Human Anti-CD19 Recombinant Antibody (HPAB-0367-FY) | Human | Human | ELISA; Inhib; FC; WB |
Creative Quality Control
We are dedicated to advancing biopharmaceutical research and development through to commercialization, with a strong emphasis on customer satisfaction. Our core values center on delivering top-tier product quality to meet the specific needs of our clients. To ensure the highest standards, we have implemented a robust quality management system. By consistently exceeding customer expectations, we aim to streamline the drug development process, helping our clients save valuable time.
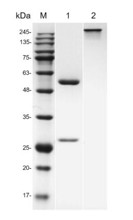 Fig.1 SDS-PAGE analysis of anti-CD19 antibody
Fig.1 SDS-PAGE analysis of anti-CD19 antibody
(Cat# TAB-431CQ, Creative Biolabs).
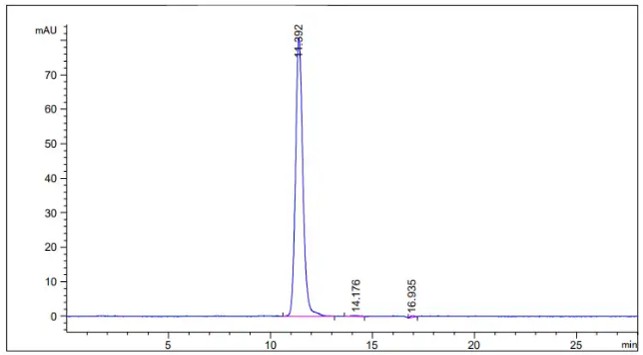 Fig.2 SEC-HPLC analysis of anti-CD19 antibody
Fig.2 SEC-HPLC analysis of anti-CD19 antibody
(Cat# HPAB-0418LY, Creative Biolabs).
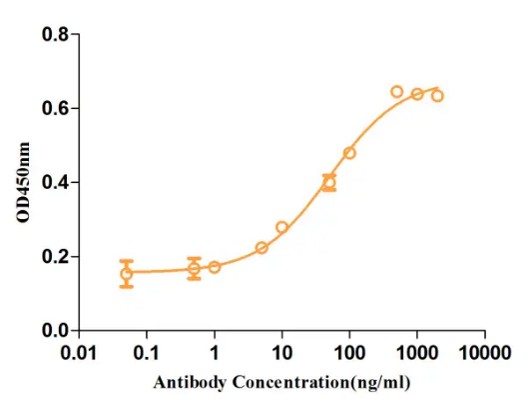 Fig.3 ELISA analysis of anti-CD19 antibody
Fig.3 ELISA analysis of anti-CD19 antibody
(Cat# TAB-108CL, Creative Biolabs).
Customer Reviews

Anti-Human CD19 Recombinant Antibody (CAT#: TAB-431CQ)

Human Anti-CD19 Recombinant Antibody (HPAB-0418LY) (CAT#: HPAB-0418LY)

Anti-Human CD19 Recombinant Antibody (TAB-108CL) (CAT#: TAB-108CL)
rAb Production
With years of experience in recombinant antibody production and optimization, we pride ourselves on offering exceptional service and delivering high-quality recombinant antibodies within the shortest possible time. Our commitment is to ensure that each product meets the highest standards of excellence.
Featured Anti-CD19 Recombinant Antibody Production Platforms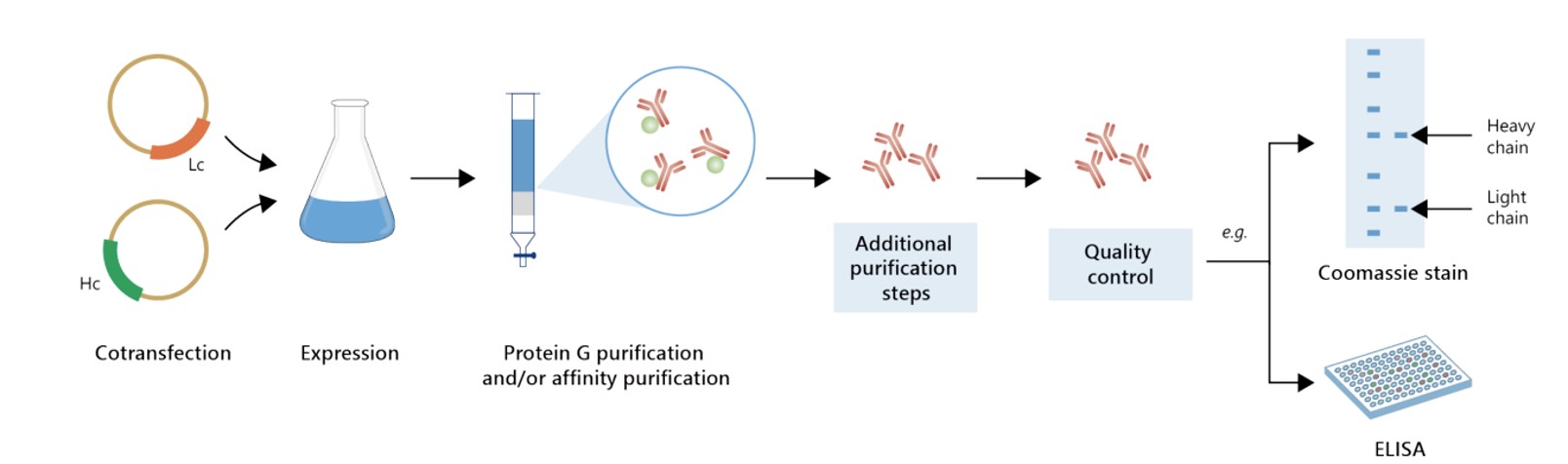
Fig.4 Milligram-scale recombinant antibody production.
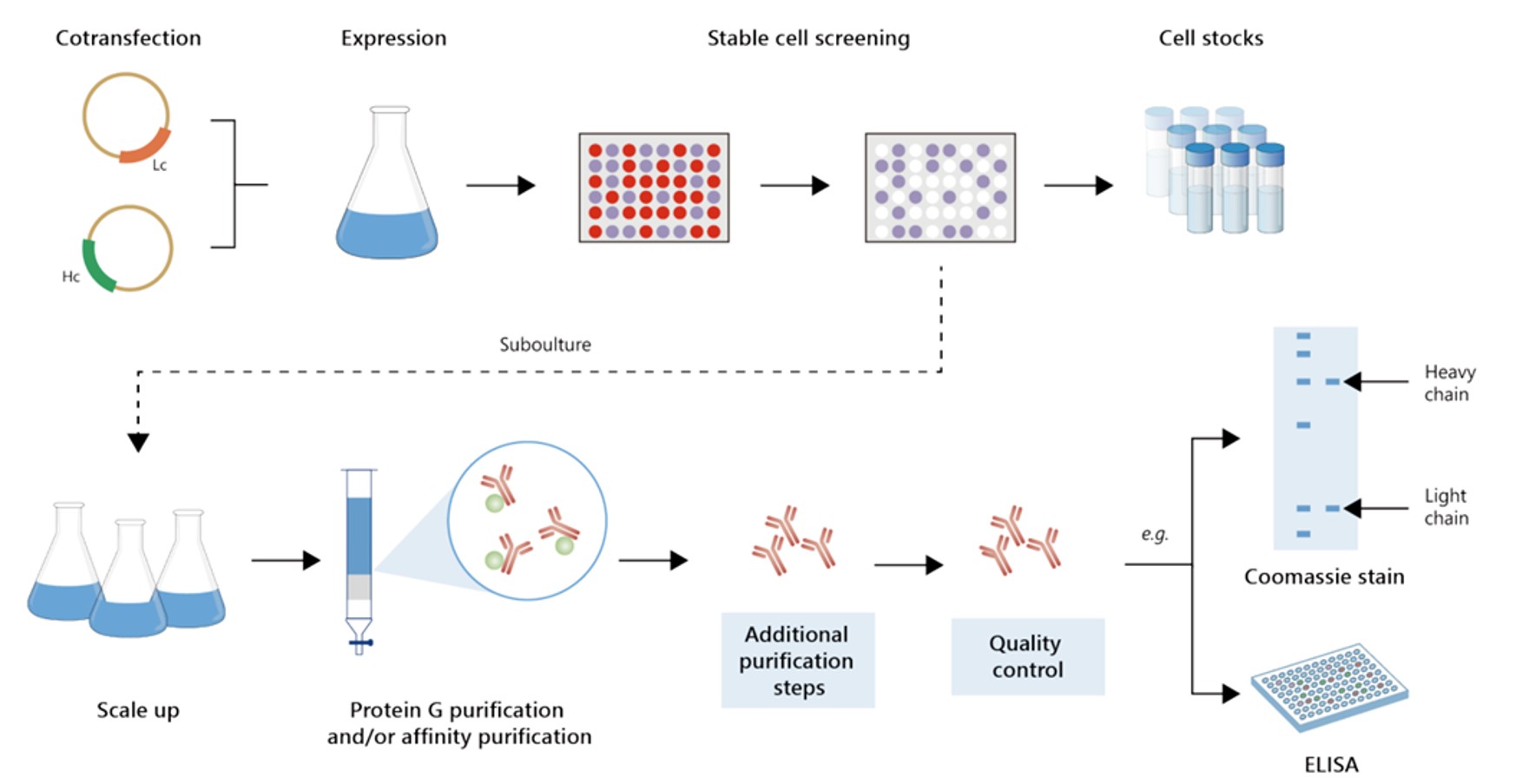 Fig.5 Gram-scale recombinant antibody production.
Fig.5 Gram-scale recombinant antibody production.
rAb Modalities
Creative Biolabs is committed to providing researchers with a comprehensive selection of cutting-edge, high-quality recombinant antibodies in diverse formats. Our expertise in antibody engineering spans a variety of advanced techniques, and we offer exceptional custom engineering services to meet your unique requirements.
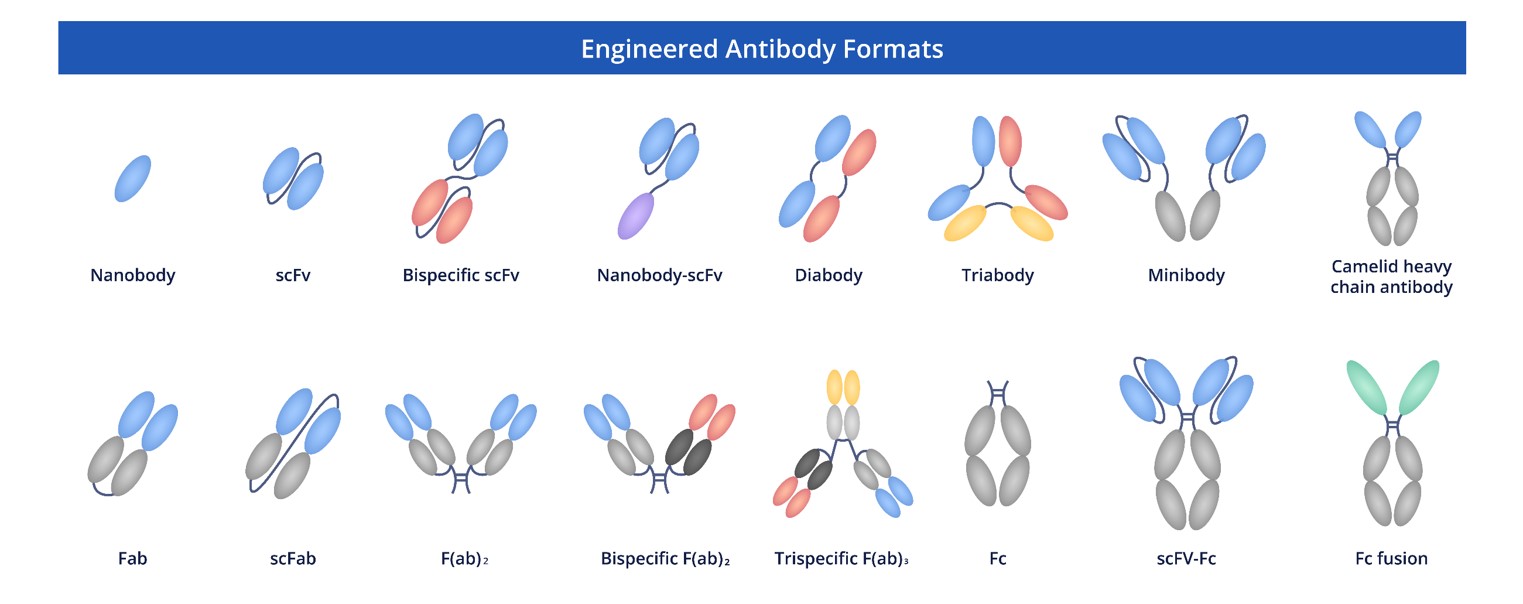 Fig.6 Full Length Anti-CD19 Recombinant Antibody Production and Modalities.
Fig.6 Full Length Anti-CD19 Recombinant Antibody Production and Modalities.
Drug Information Targeting CD19
Table 1. Therapeutic approaches targeting CD19 in clinical development.
| Research phase | Company | Classification | Indications | Details |
| Launched - 2023 | Immunoadoptive Cell | Biologics |
B-cell leukemia; Lymphocytic leukemia |
A CAR-T therapy targeting CD19, developed by the Indian Institute of Technology and Tata Memorial Center, was approved in India in 2023 for the treatment of B-cell lymphomas and B-cell acute lymphoblastic leukemia in patients who have not responded to prior treatments. |
| Launched - 2021 | JW Therapeutics | Biologics | B-cell acute lymphocytic leukemia | Developed by JW Therapeutics, this anti-CD19 CAR-T therapy has received approval in China for the treatment of relapsed or refractory large B-cell lymphoma and follicular lymphoma. |
| Launched - 2021 | ADC Therapeutics | Drug Conjugates |
Cancer; Lymphoma, B-cell |
This antibody-drug conjugate targeting CD19 has been approved in the U.S. and E.U. for relapsed or refractory large B-cell lymphoma and high-grade B-cell lymphoma. |
| Launched - 2021 | Bristol-Myers Squibb | Biologics | Acute lymphocytic leukemia | Developed by Bristol-Myers Squibb, this anti-CD19 CAR-T therapy is approved for relapsed or refractory large B-cell lymphoma, follicular lymphoma, and other subtypes across multiple regions. It is currently being studied in other types of non-Hodgkin's lymphoma, with ongoing regulatory reviews for additional indications. |
| Launched - 2020 | Kite Pharma | Biologics |
B-cell acute lymphocytic leukemia; Chronic lymphocytic leukemia |
Developed by Kite Pharma, this anti-CD19 CAR-T therapy is approved for relapsed or refractory mantle cell lymphoma and B-cell precursor acute lymphoblastic leukemia. It is also undergoing trials in pediatric patients and for rare B-cell malignancies. |
| Launched - 2020 | Amgen | Biologics |
Autoimmune disease; Cancer; Chronic lymphocytic leukemia |
Developed by Viela Bio, this anti-CD19 monoclonal antibody is approved for the treatment and relapse prevention of neuromyelitis optica spectrum disorder. It is currently undergoing phase III trials for myasthenia gravis, IgG4-related disease, and systemic sclerosis. |
| Launched - 2020 | Incyte | Biologics |
Autoimmune disease; B-cell acute lymphocytic leukemia |
This Fc-engineered anti-CD19 monoclonal antibody, developed by Incyte, is approved for relapsed or refractory diffuse large B-cell lymphoma when used in combination with lenalidomide. Ongoing phase III trials are investigating its use for follicular lymphoma and marginal zone lymphoma, and it has been licensed to several companies worldwide. |
| Launched - 2017 | Novartis | Biologics |
Acute lymphocytic leukemia; B-cell acute lymphocytic leukemia |
Developed by the University of Pennsylvania and Novartis, this CD19-targeted CAR-T therapy is approved for several B-cell malignancies, including relapsed or refractory ALL, DLBCL, and follicular lymphoma. It is currently being tested in clinical trials for chronic lymphocytic leukemia and multiple myeloma. |
| Launched - 2017 | Adimab | Biologics |
B-cell acute lymphocytic leukemia; Cancer |
Developed by Kite Pharma, this CD19-targeted CAR-T therapy is approved for relapsed or refractory DLBCL, PMBCL, follicular lymphoma, and transformed follicular lymphoma. Ongoing trials are exploring its use for other B-cell malignancies, and regulatory filings are underway for additional indications in multiple regions. |
| Launched - 2017 | Reva Medical | Others | Coronary artery disease | A sirolimus-eluting bioresorbable coronary scaffold developed by Reva Medical was launched in 2017 in Europe for the treatment of coronary artery disease. |
| Launched - 2015 | Micell Technologies | Others | Coronary artery disease | Developed by Micell Technologies, this sirolimus-eluting stent was launched in Europe in 2015 for the treatment of coronary artery disease. It is currently in phase III trials in China for de novo lesions in native coronary arteries. |
| Launched - 2014 | Amgen | Biologics |
Acute lymphocytic leukemia; Chronic lymphocytic leukemia |
Developed by Amgen, this bispecific antibody targeting CD19 and CD3 was first approved in the U.S. in 2014 for relapsed or refractory B-precursor ALL. It has since received global approval for various B-cell precursor ALL indications and is undergoing ongoing clinical development for other B-cell malignancies. |
If you need further details about the CD19 target, don't hesitate to contact us by phone or email. Our expert team is always ready to provide assistance and answer any questions you may have, offering the support you need to ensure the success of your research.


 Primary Immunodeficiency
Primary Immunodeficiency