Mouse Anti-CD4 Recombinant Antibody (clone Leu3a)
CAT#: FAMAB-0048CQ
Recombinant Mouse Antibody clone Leu3a, which is specific to CD4. This product is a monoclonal anti-CD4 antibody which inhibits the human immunodeficiency virus (HIV) gp120 binding to CD4.






Specifications
- Host Species
- Mouse
- Type
- Mouse IgG1, κ
- Specificity
- Human CD4
- Species Reactivity
- Human
- Clone
- Leu3a
- Applications
- ELISA, FC, Block, FuncS
Product Property
- Purity
- >95% as determined by SDS-PAGE and HPLC analysis
- Concentration
- Please refer to the vial label for the specific concentration.
- Buffer
- PBS
- Preservative
- No preservatives
- Storage
- Centrifuge briefly prior to opening vial. Store at +4°C short term (1-2 weeks). Aliquot and store at -20°C long term. Avoid repeated freeze/thaw cycles.
Applications
- Application Notes
- This antibody has been tested for use in Flow Cytometry, Blocking and Functional Assay.
Target
Customer Review
There are currently no Customer reviews or questions for FAMAB-0048CQ. Click the button above to contact us or submit your feedback about this product.



Q&As
-
How does antibody aggregation affect the outcomes of my studies?
A: Results that are erroneous and non-specific can arise from aggregated antibodies.
-
How do I decide between a monoclonal antibody made from mice or from rabbits?
A: Take into account the particular application, the possibility of host immunogenicity, and the availability of secondary reagents.
-
What function does a normalizer serve in an ELISA test?
A: A normalizer maintains uniform assay conditions, which aids in adjusting for well-to-well variability.
View the frequently asked questions answered by Creative Biolabs Support.
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
See other products for "Clone Leu3a"
- CAT
- Product Name
See other products for "CD4"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1067z | Mouse Anti-CD4 Recombinant Antibody (clone 34D6) | FC, FuncS, ICC, IF, IP, WB | Mouse IgG2b, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-039 | Anti-Human CD4 Recombinant Antibody (Zanolimumab) | Neut, ELISA, IF, IP, FuncS, FC, IHC | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-260 | Anti-Human CD4 Recombinant Antibody (Tregalizumab) | WB, ELISA, FC, IP, FuncS, IF, Neut | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-164 | Anti-Human CD4 Recombinant Antibody (TAB-164) | WB, FuncS, IF, Neut, ELISA, FC, IP | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-171 | Anti-Human CD4 Recombinant Antibody (TAB-171) | FuncS, IF, Neut, ELISA, FC, IP, ICC | IgG1 - lambda |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-732 | Anti-CD4 Recombinant Antibody (Clenoliximab) | IF, IP, Neut, FuncS, ELISA, FC, ICC | IgG4 - lambda |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-107 | Anti-Human CD4 Recombinant Antibody (TAB-107) | IF, IP, Neut, FuncS, ELISA, FC, ICC | IgG4 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-146 | Anti-Human CD4 Recombinant Antibody (TAB-146) | FuncS, IF, Neut, ELISA, FC, IP, ICC | IgG4 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-G078E | Anti-CD4 immunotoxin M-T151 (IgG)-PE | Cytotoxicity assay, Function study |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-G078R | Anti-CD4 immunotoxin M-T151 (IgG)-RTA | Cytotoxicity assay, Function study |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-033 | Human Anti-CD4 Recombinant Antibody (clone 17b) | ELISA, Block, FC | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-034 | Mouse Anti-CD4 Recombinant Antibody (PABL-034) | Neut, ELISA, FuncS | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-035 | Mouse Anti-CD4 Recombinant Antibody (clone Q425) | WB, ELISA, FuncS | Mouse IgG, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-033 | Human Anti-CD4 Recombinant Antibody (clone 17b); scFv Fragment | WB, ELISA, FuncS | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-034 | Mouse Anti-CD4 Recombinant Antibody scFv Fragment (PSBL-034) | Neut, ELISA, FuncS | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-035 | Mouse Anti-CD4 Recombinant Antibody (clone Q425); scFv Fragment | WB, ELISA, FuncS | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-033 | Human Anti-CD4 Recombinant Antibody (clone 17b); Fab Fragment | WB, ELISA, FuncS | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-034 | Mouse Anti-CD4 Recombinant Antibody Fab Fragment (PFBL-034) | Neut, ELISA, FuncS | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-035 | Mouse Anti-CD4 Recombinant Antibody (clone Q425); Fab Fragment | WB, ELISA, FuncS | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-426 | Human Anti-CD4 Recombinant Antibody (clone 576) | WB, ELISA, FuncS | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-423 | Human Anti-CD4 Recombinant Antibody (clone 576); Fab Fragment | WB, ELISA, FuncS | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-424 | Human Anti-CD4 Recombinant Antibody (clone Hu5A8); Fab Fragment | Block, Neut | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-423 | Human Anti-CD4 Recombinant Antibody (clone 576); scFv Fragment | WB, ELISA, FuncS | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-424 | Human Anti-CD4 Recombinant Antibody (clone Hu5A8); scFv Fragment | Block, Neut | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-160LC | Anti-Human CD4 Recombinant Antibody (OKT4A) | ELISA, WB |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-161LC | Anti-Human CD4 Recombinant Antibody (gOKT4A-4) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-162LC | Anti-Human CD4 Recombinant Antibody | ELISA | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-166LC | Anti-Human CD4 Recombinant Antibody (M-T412) | ELISA, Inhib |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-167LC | Anti-Human CD4 Recombinant Antibody (cM-T412) | ELISA, Inhib | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-168LC | Anti-Human CD4 Recombinant Antibody (TRX1) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-169LC | Rat Anti-CD4 Recombinant Antibody (TAB-169LC) | ELISA, FC, SPR | Rat IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-173LC | Anti-Human CD4 Recombinant Antibody (IgA F425-Alg8) | ELISA, Inhib |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-174LC | Mouse Anti-CD4 Recombinant Antibody (TAB-174LC) | ELISA, FC, IHC, Neut, FuncS | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-025LC | Recombinant Anti-Human CD4 Antibody (Fab glycosylation) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-351CQ | Mouse Anti-CD4 Recombinant Antibody (clone 10C12) | ELISA, WB, IF, Block | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-352CQ | Mouse Anti-CD4 Recombinant Antibody (clone 13B8.2) | Block, ELISA, FC, IHC-Fr, WB | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-353CQ | Mouse Anti-CD4 Recombinant Antibody (clone CBL976) | FC, Neut, IF, IHC | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-354CQ | Recombinant Mouse Anti-CD4 Antibody (CT-4) | Neut, FC, IHC, IHC-Fr, IHC-P, IP | IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-355CQ | Mouse Anti-CD4 Recombinant Antibody (clone RPA-T4) | FC, CyTOF, Block, IHC | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-356CQ | Mouse Anti-CD4 Recombinant Antibody (clone 3-4F4) | Neut, FC, IHC, IHC-Fr, IP, ICC | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-357CQ | Rat Anti-CD4 Recombinant Antibody (clone MAB0810) | Block, Depletion, Stim | Rat IgG2b |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-358CQ | Rat Anti-CD4 Recombinant Antibody (clone RM4-5) | FC, CyTOF, Depletion, Block, IHC | Rat IgG2a, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-0560 | Hi-Affi™ Rabbit Anti-CD4 Recombinant Antibody (clone DS560AB) | IHC-P | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-039 | Afuco™ Anti-CD4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-039) | Neut, ELISA, IF, IP, FuncS, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-164 | Afuco™ Anti-CD4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-164) | FuncS, IF, Neut, ELISA, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-260 | Afuco™ Anti-CD4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-260) | ELISA, FC, IP, FuncS, IF | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-171 | Afuco™ Anti-CD4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-171) | FuncS, IF, Neut, ELISA, FC, IP | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-107 | Afuco™ Anti-CD4 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-107) | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0724-YC759 | AbPlus™ Anti-Cd4 Magnetic Beads (VS-0724-YC759) | IP, Protein Purification |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0924-YC114 | Rat Anti-CD4 Recombinant Antibody Hexamer (VS-0924-YC114), CDC Enhanced | ELISA, FC, CDC | Antibody hexamer |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0924-YC115 | Human Anti-CD4 Recombinant Antibody Hexamer (VS-0924-YC115), CDC Enhanced | ELISA, FC, ADCC, CDC, SPR | Antibody hexamer |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0924-YC116 | Mouse Anti-CD4 Recombinant Antibody Hexamer (VS-0924-YC116), CDC Enhanced | WB, ELISA, IF, CDC | Antibody hexamer |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1024-XY95 | Mouse Anti-NHP CD4 Recombinant Antibody (clone RIV7) | IF, FC | Mouse IgG2a, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY48 | CytoStream™ Mouse Anti-CD4 Recombinant Antibody (clone OKT4) | FC | Mouse IgG2b, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY49 | CytoStream™ Mouse Anti-CD4 Recombinant Antibody (clone OX-35) | FC | Mouse IgG2a, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY50 | CytoStream™ Rat Anti-CD4 Recombinant Antibody (clone RM4-4) | FC | Rat IgG2b, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY51 | CytoStream™ Mouse Anti-CD4 Recombinant Antibody (clone KEN-4) | FC, Block, IHC, IP | Mouse IgG2a, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-FY64 | Human Anti-CD4 (clone TRX1) scFv-Fc Chimera | ELISA | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0425-YC755 | Recombinant Anti-CD4 Vesicular Antibody, EV Displayed (VS-0425-YC755) | ELISA, FC, Neut, Cell-uptake |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY1178 | Anti-CD4 Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY1179 | Anti-Mouse CD4 Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY1180 | Anti-Human CD4 Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC204 | Recombinant Anti-CD4 (Domain 1 x Domain 2) Biparatopic Antibody, Tandem scFv | ELISA, FC, ICC, IF, IP, Neut | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC56 | SmartAb™ Recombinant Anti-CD4 pH-dependent Antibody (Clone Zanolimumab) | Neut, ELISA, IF, IP, FC, IHC | Human IgG1 kappa |
Popular Products

Application: Neut, ELISA, IF, IP, FuncS, FC, IHC

Application: WB, FC, IP, ELISA, Neut, FuncS, IF

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC

Application: WB, IF, IP, Neut, FuncS, ELISA, FC

Application: IF, IP, Neut, FuncS, ELISA, FC, ICC

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: WB, IP, IF, FuncS, FC, Neut, ELISA

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC

Application: IF, IP, Neut, FuncS, ELISA, FC, WB

Application: ELISA, FC, IP, FuncS, IF, Neut, ICC
-2-1.png)
Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: ELISA, WB, BLI, SPR
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.









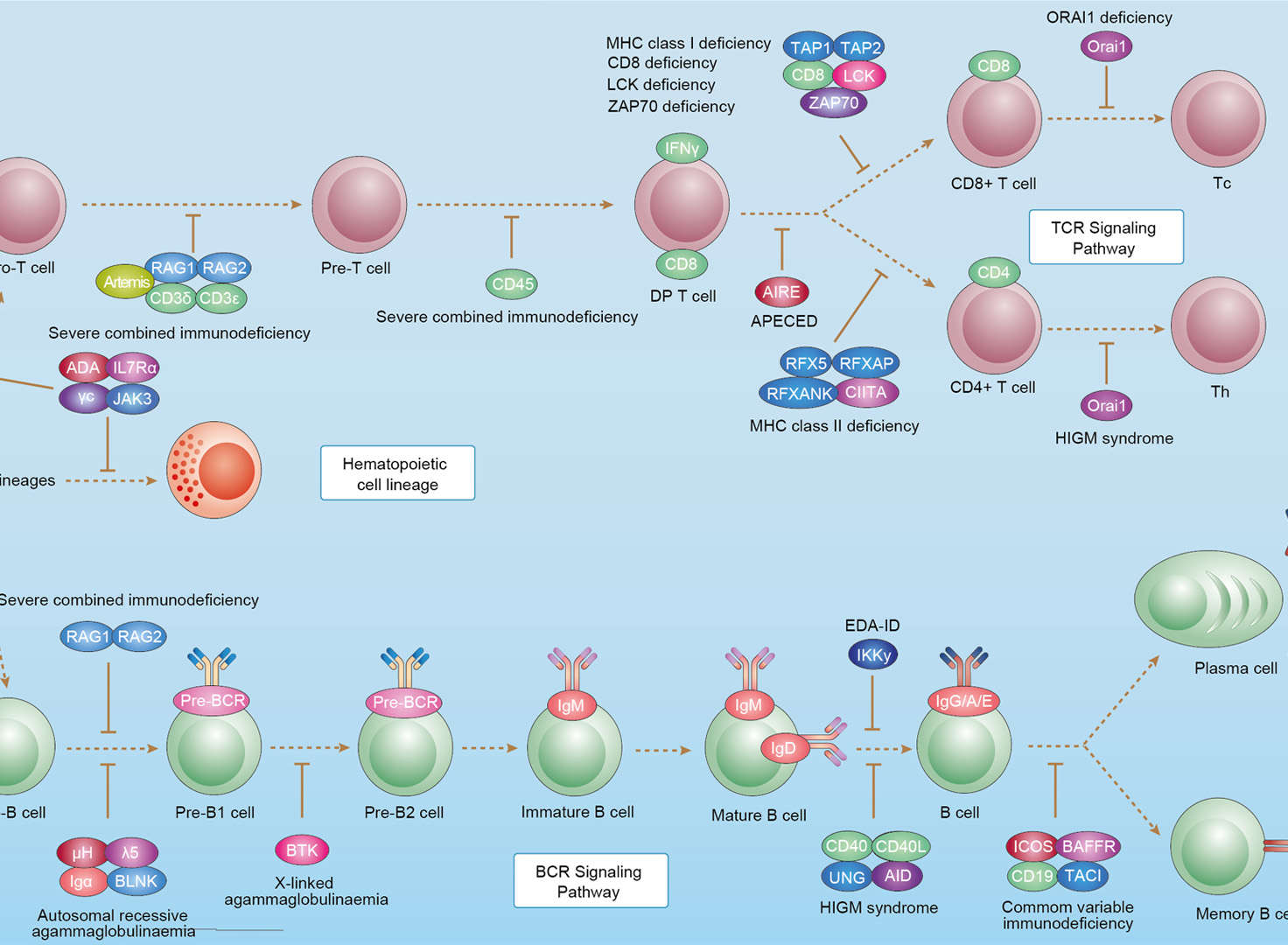 Primary Immunodeficiency
Primary Immunodeficiency








