 Loading...
Loading...

Anti-TNFRSF9 Recombinant Antibody Products
 Loading...
Loading...Anti-TNFRSF9 Products
-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG4 - kappa
- Application: ELISA, FC, IP, FuncS, IF, Neut, ICC
-
- Derivation: Human
- Species Reactivity: Human
- Type: IgG2, λ
- Application: ELISA, IHC, FC, IP, IF, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0253-CN) (HPAB-0253-CN)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, FC
-
- Species Reactivity: Human
- Type: ADCC enhanced antibody
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0256-CN) (HPAB-0256-CN)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0257-CN) (HPAB-0257-CN)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0258-CN) (HPAB-0258-CN)
-
- Species Reactivity: Human, Cynomolgus
- Type: Human IgG1
- Application: Block, ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0259-CN) (HPAB-0259-CN)
-
- Species Reactivity: Human, Cynomolgus
- Type: Human IgG4
- Application: Block, ELISA, FC
-
- Species Reactivity: Human, Chimpanzee, Baboon, Cynomolgus, Rhesus
- Type: Mouse IgG1, κ
- Application: ELISA, IP, FC, FuncS
-
- Species Reactivity: Mouse
- Type: Rat IgG
- Application: FuncS
-
- Species Reactivity: Human
- Type: Human IgG2
- Application: ELISA, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (TAB-0613CLV) (TAB-0613CLV)
-
- Species Reactivity: Human
- Type: Chimeric (mouse/human) IgG1
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody (TAB-0614CLV) (TAB-0614CLV)
-
- Species Reactivity: Human
- Type: Chimeric (mouse/human) IgG1
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody (TAB-0611CLV) (TAB-0611CLV)
-
- Species Reactivity: Human
- Type: Humanized IgG1
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (TAB-0611CLV-F(E)) (TAB-0611CLV-F(E))
-
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody (TAB-0612CLV) (TAB-0612CLV)
-
- Species Reactivity: Human
- Type: Humanized IgG4
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (TAB-0612CLV-F(E)) (TAB-0612CLV-F(E))
-
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (TAB-0613CLV-F(E)) (TAB-0613CLV-F(E))
-
- Species Reactivity: Human
- Type: Chimeric (mouse/human) Fab
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (TAB-0614CLV-F(E)) (TAB-0614CLV-F(E))
-
- Species Reactivity: Human
- Type: Chimeric (mouse/human) Fab
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (TAB-0611CLV-S(P)) (TAB-0611CLV-S(P))
-
- Species Reactivity: Human
- Type: Humanized scFv
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (TAB-0612CLV-S(P)) (TAB-0612CLV-S(P))
-
- Species Reactivity: Human
- Type: Humanized scFv
- Application: ELISA
- Mouse Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (TAB-0613CLV-S(P)) (TAB-0613CLV-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
- Mouse Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (TAB-0614CLV-S(P)) (TAB-0614CLV-S(P))
-
- Species Reactivity: Human
- Type: Mouse scFv
- Application: ELISA
-
- Species Reactivity: Human
- Type: Mouse antibody
- Application: FC
- Rat Anti-Tnfrsf9 Recombinant Antibody (clone CBL332) (NEUT-2167CQ)
-
- Species Reactivity: Mouse
- Type: Rat IgG2b
- Application: Block, WB
- Hamster Anti-Tnfrsf9 Recombinant Antibody (clone 17B5) (NEUT-2168CQ)
-
- Species Reactivity: Mouse
- Type: Hamster IgG
- Application: FC, Block
- Rat Anti-Tnfrsf9 Recombinant Antibody (NEUT-2169CQ) (NEUT-2169CQ)
-
- Species Reactivity: Mouse
- Type: Rat IgG2
- Application: Neut, WB
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-N0400-YC) (HPAB-N0400-YC)
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human IgG
- Application: ELISA, FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-N0401-YC) (HPAB-N0401-YC)
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human IgG
- Application: ELISA, FC, FuncS
- Mouse Anti-TNFRSF9 Recombinant Antibody (HPAB-N0402-YC) (HPAB-N0402-YC)
-
- Species Reactivity: Human, Monkey
- Type: Mouse IgG
- Application: FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-N0403-YC) (HPAB-N0403-YC)
-
- Species Reactivity: Human, Monkey
- Type: Human IgG4
- Application: FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0809LY) (HPAB-0809LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0810LY) (HPAB-0810LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0811LY) (HPAB-0811LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0260-CN) (HPAB-0260-CN)
-
- Species Reactivity: Human, Cynomolgus
- Type: Human IgG1
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0261-CN) (HPAB-0261-CN)
-
- Species Reactivity: Human, Cynomolgus
- Type: Human IgG1
- Application: ELISA, FC
- Mouse Anti-TNFRSF9 Recombinant Antibody (clone 4B4-1-1) (HPAB-0262-CN)
-
- Species Reactivity: Human
- Type: Mouse IgG1, κ
- Application: ELISA, FC
-
- Species Reactivity: Human
- Type: Human IgG2
- Application: ELISA
- Rat Anti-Tnfrsf9 Agonistic Antibody (VS-0724-XY51) (VS-0724-XY51)
-
- Species Reactivity: Mouse
- Type: Rat IgG2a
- Application: Agonistic assays
- Rabbit Anti-TNFRSF9 Agonistic Antibody (VS-0724-XY52) (VS-0724-XY52)
-
- Species Reactivity: Human
- Type: Rabbit IgG
- Application: FC, Agonistic assays, CyTOF®
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, FC, FuncS
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized IgG
- Application: ELISA, FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-N0282-YC) (HPAB-N0282-YC)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: FC
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0254-CN) (HPAB-0254-CN)
-
- Species Reactivity: Human
- Type: Human IgG
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0255-CN) (HPAB-0255-CN)
-
- Species Reactivity: Human, Cynomolgus
- Type: Human IgG4, λ
- Application: ELISA, FC
- Rat Anti-TNFRSF9 Recombinant Antibody (clone LOB12.3) (HPAB-0670YY)
-
- Species Reactivity: Mouse
- Type: Rat IgG2a
- Application: FC, Inhib
- Human Anti-TNFRSF9 Recombinant Antibody (HPAB-0812LY) (HPAB-0812LY)
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human IgG1
- Application: FC, FuncS
-
- Derivation: Mouse
- Species Reactivity: Human
- Type: Mouse IgG1
- Application: ELISA, FC, IP, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0820LY-S(P)) (HPAB-0820LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-2828LY-S(P)) (HPAB-2828LY-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, FC
- Rat Anti-TNFRSF9 Recombinant Antibody (clone LOB12.3); scFv Fragment (HPAB-0670YY-S(P))
-
- Species Reactivity: Mouse
- Type: Rat scFv
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0255-CN-S(P)) (HPAB-0255-CN-S(P))
-
- Species Reactivity: Human, Cynomolgus
- Type: Human scFv
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0254-CN-S(P)) (HPAB-0254-CN-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody (clone Hz4B4-1); Fab Fragment (HPAB-0011-YJ-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0815LY-S(P)) (HPAB-0815LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (clone Hz4B4-2); Fab Fragment (HPAB-0012-YJ-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0816LY-S(P)) (HPAB-0816LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody (clone hu39E3.G4); Fab Fragment (HPAB-0033-YJ-F(E))
-
- Derivation: Humanized
- Species Reactivity: Human
- Type: Humanized Fab
- Application: Costim, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0817LY-S(P)) (HPAB-0817LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0818LY-S(P)) (HPAB-0818LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-2826LY-S(P)) (HPAB-2826LY-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0819LY-S(P)) (HPAB-0819LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-2827LY-S(P)) (HPAB-2827LY-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-N0400-YC-S(P)) (HPAB-N0400-YC-S(P))
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human scFv
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-N0401-YC-S(P)) (HPAB-N0401-YC-S(P))
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human scFv
- Application: ELISA, FC
- Mouse Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-N0402-YC-S(P)) (HPAB-N0402-YC-S(P))
-
- Species Reactivity: Human, Monkey
- Type: Mouse scFv
- Application: FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-N0403-YC-S(P)) (HPAB-N0403-YC-S(P))
-
- Species Reactivity: Human, Monkey
- Type: Human scFv
- Application: FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0809LY-F(E)) (HPAB-0809LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0810LY-F(E)) (HPAB-0810LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0811LY-F(E)) (HPAB-0811LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0812LY-F(E)) (HPAB-0812LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0813LY-F(E)) (HPAB-0813LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0814LY-F(E)) (HPAB-0814LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0815LY-F(E)) (HPAB-0815LY-F(E))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human Fab
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-2826LY-F(E)) (HPAB-2826LY-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-2827LY-F(E)) (HPAB-2827LY-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-2828LY-F(E)) (HPAB-2828LY-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-N0400-YC-F(E)) (HPAB-N0400-YC-F(E))
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-N0401-YC-F(E)) (HPAB-N0401-YC-F(E))
-
- Species Reactivity: Human, Cynomolgus monkey
- Type: Human Fab
- Application: ELISA, FC
- Mouse Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-N0402-YC-F(E)) (HPAB-N0402-YC-F(E))
-
- Species Reactivity: Human, Monkey
- Type: Mouse Fab
- Application: FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-N0403-YC-F(E)) (HPAB-N0403-YC-F(E))
-
- Species Reactivity: Human, Monkey
- Type: Human Fab
- Application: FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0814LY-S(P)) (HPAB-0814LY-S(P))
-
- Derivation: Human
- Species Reactivity: Human
- Type: Human scfv
- Application: FC, FuncS
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0253-CN-F(E)) (HPAB-0253-CN-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0254-CN-F(E)) (HPAB-0254-CN-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0255-CN-F(E)) (HPAB-0255-CN-F(E))
-
- Species Reactivity: Human, Cynomolgus
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0256-CN-F(E)) (HPAB-0256-CN-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0257-CN-F(E)) (HPAB-0257-CN-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0258-CN-F(E)) (HPAB-0258-CN-F(E))
-
- Species Reactivity: Human, Cynomolgus
- Type: Human Fab
- Application: Block, ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0259-CN-F(E)) (HPAB-0259-CN-F(E))
-
- Species Reactivity: Human, Cynomolgus
- Type: Human Fab
- Application: Block, ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0260-CN-F(E)) (HPAB-0260-CN-F(E))
-
- Species Reactivity: Human, Cynomolgus
- Type: Human Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0261-CN-F(E)) (HPAB-0261-CN-F(E))
-
- Species Reactivity: Human, Cynomolgus
- Type: Human Fab
- Application: ELISA, FC
- Mouse Anti-TNFRSF9 Recombinant Antibody (clone 4B4-1-1); Fab Fragment (HPAB-0262-CN-F(E))
-
- Species Reactivity: Human
- Type: Mouse Fab
- Application: ELISA, FC
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0053-FY-S(P)) (HPAB-0053-FY-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA
- Human Anti-TNFRSF9 Recombinant Antibody; Fab Fragment (HPAB-0053-FY-F(E)) (HPAB-0053-FY-F(E))
-
- Species Reactivity: Human
- Type: Human Fab
- Application: ELISA
-
- Scaffold Name: Z-domain of protein A
- Target: CD28
- Species Reactivity: Human
- Derivation: Phage display
- Human Anti-TNFRSF9 Recombinant Antibody; scFv Fragment (HPAB-0253-CN-S(P)) (HPAB-0253-CN-S(P))
-
- Species Reactivity: Human
- Type: Human scFv
- Application: ELISA, FC
- Anti-TNFRSF9 immunotoxin (scFv)-Sap (AGTO-G063S)
-
- Species Reactivity: Human
- Application: Cytotoxicity assay, Function study
- Human Anti-Human TNFRSF9 Recombinant Antibody (TAB-636CL)
-
- Species Reactivity: Human
- Type: Antibody
-
- Host Species: Human
- Specificity: Human
- Type: Human antibody
- Classification: Fc glycosylation/High-mannose glycosylated
- Glycosylation site: Fc region
- Glycoform (ratio): Man5 without fucose
-
- Host Species: Human
- Specificity: Human
- Type: Human antibody
- Classification: Fc glycosylation/High-mannose glycosylated
- Glycosylation site: Fc region
- Glycoform (ratio): Man5 without fucose
Can't find the products you're looking for? Try to filter in the left sidebar.Filter By Tag
Our customer service representatives are available 24 hours a day, from Monday to Sunday. Contact Us
For Research Use Only. Not For Clinical Use.
Creative Biolabs provides a comprehensive range of CD137-targeted recombinant antibodies to customers worldwide, leveraging our cutting-edge technologies. Our industry-leading resources and team provide exceptional value in moving research forward and assisting global customers in meeting their research objectives.
CD137: A Promising Target in Cancer Immunotherapy
Activation of CD137 (4-1BB /CD137/ILA), a receptor found on activated T cells and numerous other immune cells, triggers signaling pathways crucial for a strong cellular immune response. Due to its capability to regulate various immune cells and its significant role in promoting T cell survival, proliferation, and effector functions, 4-1BB has emerged as a promising target in the advancement of cancer immunotherapy.
Alternative Names
TNF Receptor Superfamily Member 9; T-Cell Antigen 4-1BB; 4-1BB; T-cell antigen ILA; Induced By Lymphocyte Activation; ILA; CD137 Antigen; CDw137; CD137; Interleukin-Activated Receptor; Homolog of Mouse Ly63; 4-1BB Ligand Receptor.
Background
Cancer-related genes, CD markers
Intracellular, Membrane (different isoforms)
Cell type enhanced (granulocytes, monocytes, T-cells, Langerhans cells, dendritic cells)
Group enriched (T-reg, memory CD8 T-cell, neutrophil, NK-cell)
Cell line enriched (HDLM-2)
Interacts with TRAF1, TRAF2 and TRAF3. Interacts with LRR-repeat protein 1/LRR-1.
Receptor
Anti-CD137 Recombinant Antibody Products
Our goal is to drive research and innovation forward by providing high-quality anti-CD137 recombinant antibodies at an outstanding value, along with exceptional technical support. Below we offer a selection of premium anti-CD137 recombinant antibodies to choose from for your meaningful needs.
| Cat. No. | Product Name | Target Species | Host Species | Applications |
| TAB-179 | Anti-Human 4-1BB Recombinant Antibody (Urelumab) | Human | Human IgG4-kappa | ELISA, FC, IP, FuncS, IF, Neut, ICC |
| TAB-457CQ | Anti-Human CD137 Recombinant Antibody | Human | Human IgG2, λ | ELISA, IHC, FC, IP, IF, FuncS |
| FN-078CQ | Rat Anti-CD137 Recombinant Antibody (clone 3H3) | Mouse | Rat IgG2a, κ | FuncS |
| HPAB-0257-CN | Human Anti-CD137 Recombinant Antibody | Human | Human IgG | FC |
| HPAB-0814LY | Human Anti-CD137 Recombinant Antibody | Human | Human IgG1 | FC, FuncS |
Creative Quality Control
We are dedicated to supporting the biopharmaceutical sector by pushing the boundaries of research and development with our superior product quality and outstanding customer support. By harnessing a robust quality management system, we are committed to enhancing the drug development journey but also delivering exceptional value to our customers, ensuring their research needs are consistently met with excellence.
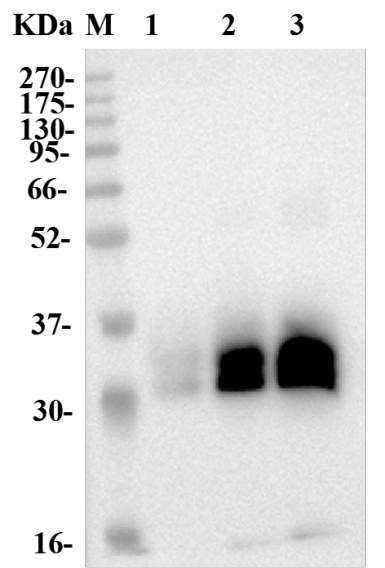 Fig.1 Anti-Human 4-1BB Therapeutic Antibody (Urelumab) in WB.
Fig.1 Anti-Human 4-1BB Therapeutic Antibody (Urelumab) in WB.
(Cat# TAB-179, Creative Biolabs).
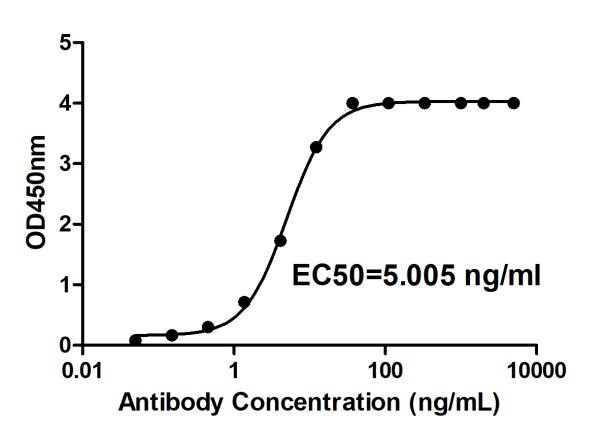 Fig.2 Anti-Human 4-1BB Therapeutic Antibody (Urelumab) in ELISA.
Fig.2 Anti-Human 4-1BB Therapeutic Antibody (Urelumab) in ELISA.
(Cat# TAB-179, Creative Biolabs).
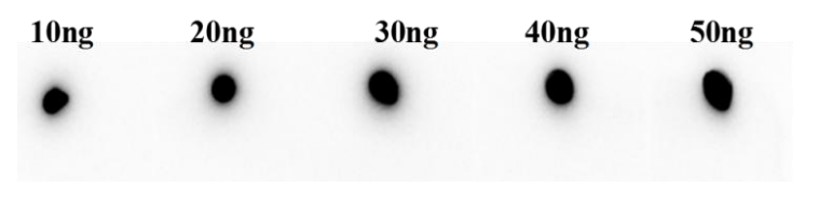 Fig.3 Anti-Human 4-1BB Therapeutic Antibody (Urelumab) in Dot Blot.
Fig.3 Anti-Human 4-1BB Therapeutic Antibody (Urelumab) in Dot Blot.
(Cat# TAB-179, Creative Biolabs).
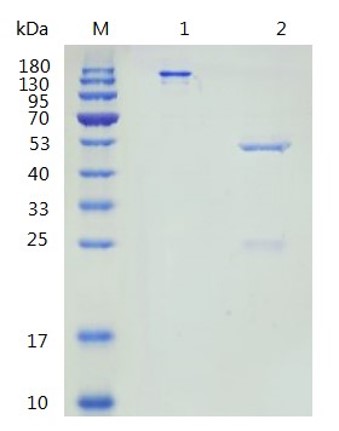 Fig.4 Anti-Human CD137 Recombinant Antibody-Low endotoxin in SDS-PAGE.
Fig.4 Anti-Human CD137 Recombinant Antibody-Low endotoxin in SDS-PAGE.
(Cat# TAB-457CQ, Creative Biolabs).
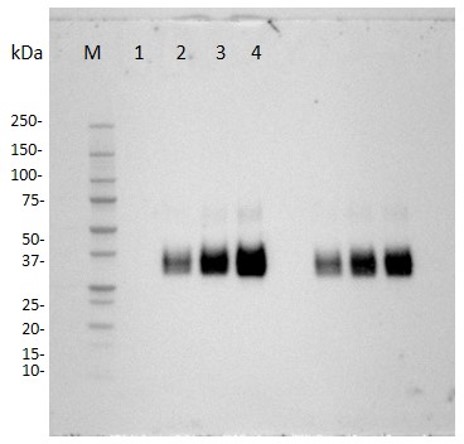 Fig.5 Anti-Human CD137 Recombinant Antibody-Low endotoxin in WB.
Fig.5 Anti-Human CD137 Recombinant Antibody-Low endotoxin in WB.
(Cat# TAB-457CQ, Creative Biolabs).
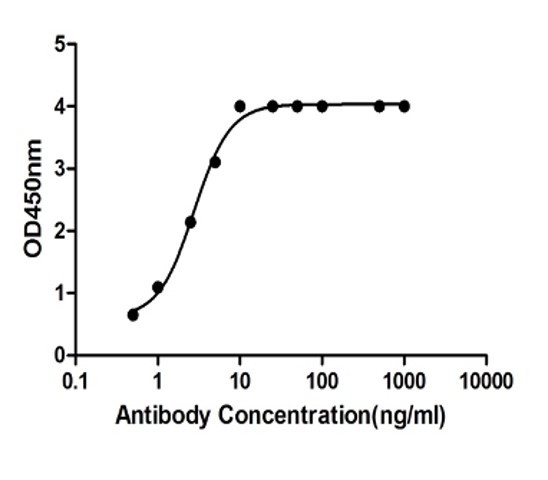 Fig.6 Anti-Human CD137 Recombinant Antibody in ELISA.
Fig.6 Anti-Human CD137 Recombinant Antibody in ELISA.
(Cat# TAB-457CQ, Creative Biolabs).
Customer Reviews

Anti-Human 4-1BB Recombinant Antibody (Urelumab) (CAT#: TAB-179)

Afuco™ Anti-CD137 ADCC Recombinant Antibody (Urelumab), ADCC Enhanced (CAT#: AFC-TAB-179)

Human Anti-CD137 Recombinant Antibody (HPAB-M0333-YC)
Anti-CD137 Recombinant Antibody Production
Leveraging our state-of-the-art technology and extensive expertise, we provide end-to-end solutions for producing high-quality anti-CD137 recombinant antibodies. From gene synthesis to large-scale manufacturing, our optimized processes ensure swift, reliable, and cost-effective antibody production tailored to your needs, all backed by over 5 years of industry experience.
Featured Anti-CD137 Recombinant Antibody Production Platforms 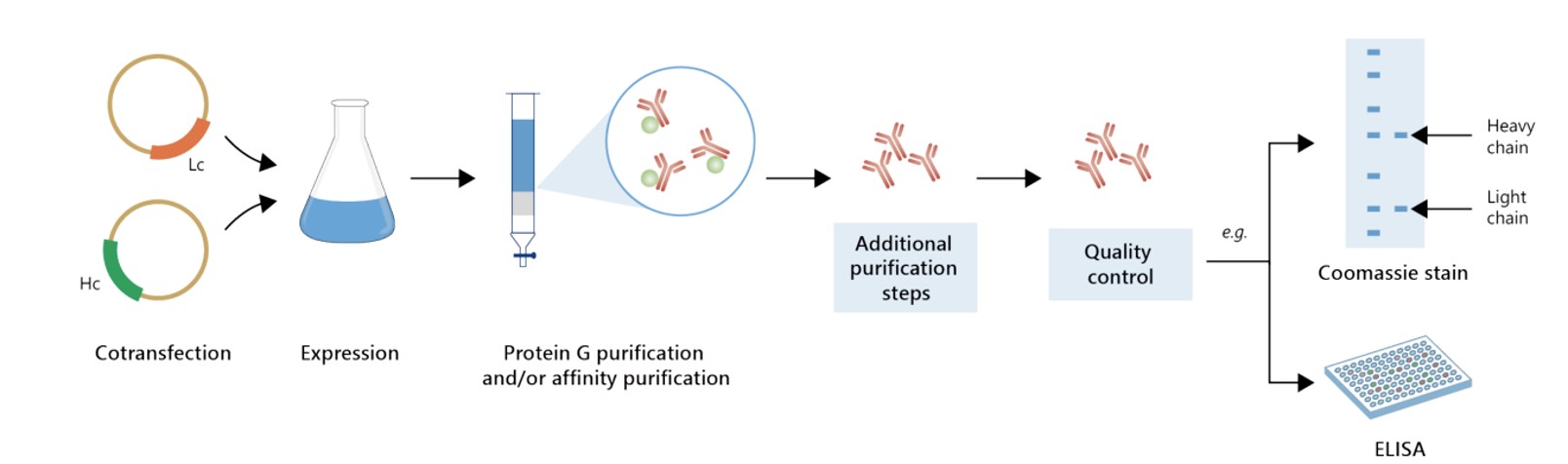
Fig.7 Milligram-scale anti-CD137 recombinant antibody production.
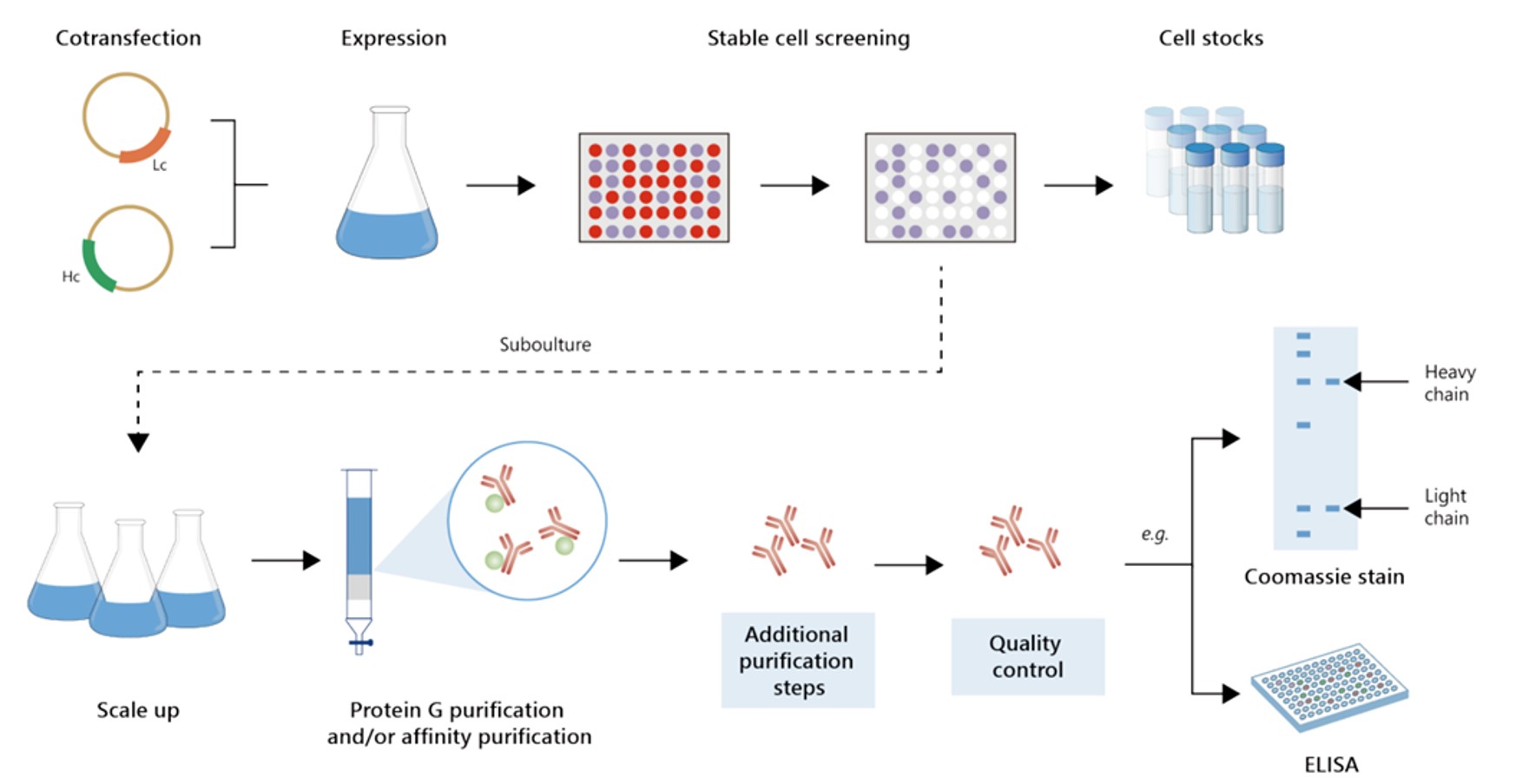 Fig.8 Gram-scale anti-CD137 recombinant antibody production.
Fig.8 Gram-scale anti-CD137 recombinant antibody production.
Anti-CD137 Recombinant Antibody Modalities
Creative Biolabs specializes in providing groundbreaking recombinant antibodies tailored for the research community. Our comprehensive portfolio includes anti-CD137 antibodies in multiple formats, including full-length antibodies, Fab fragments, and single-chain variable fragments (scFv). With our deep expertise and proven proficiency in antibody engineering, we are well-equipped to offer bespoke solutions designed to accommodate your unique research requirements.
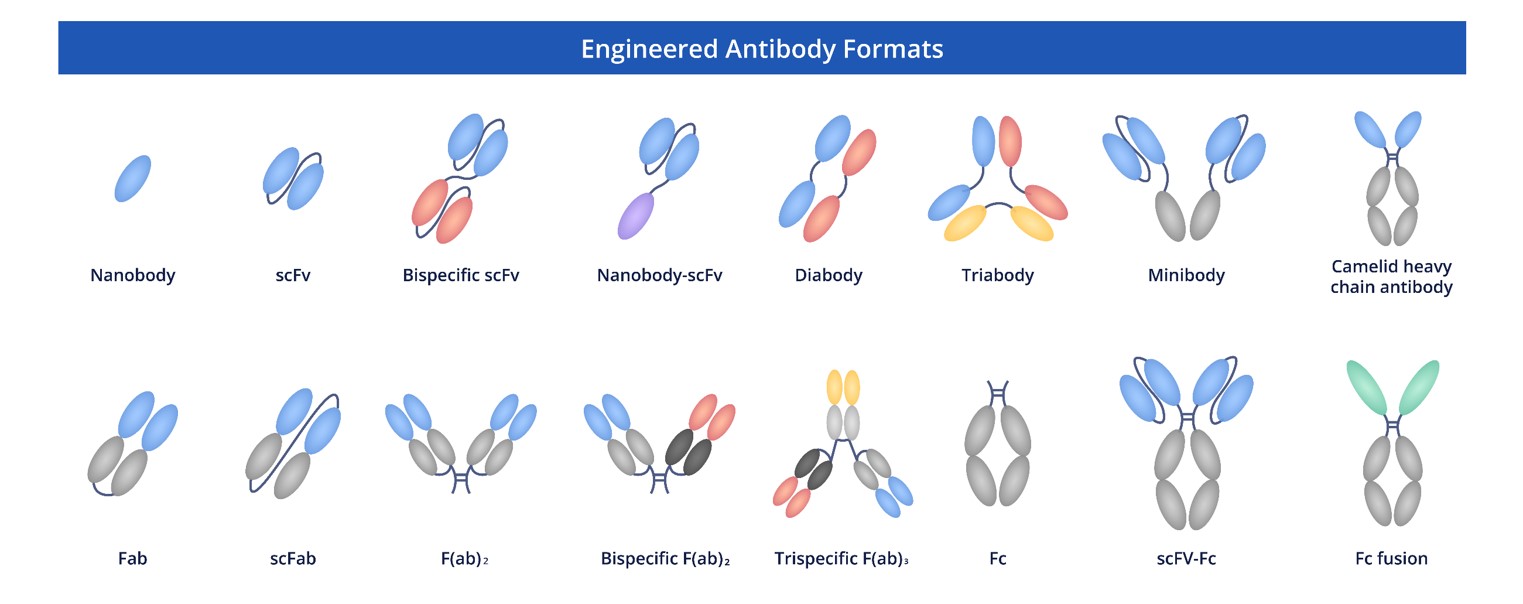 Fig.9 Full-Length Anti-CD137 Recombinant Antibody Production and Modalities.
Fig.9 Full-Length Anti-CD137 Recombinant Antibody Production and Modalities.
CD137-Targeted Drug Information
Table 1. CD137-targeted drug information.
| Company | Company | Condition | Indication |
| Launched - 2023 | Immunoadoptive Cell Therapy | Lymphoma, B-cell | Treatment of B-cell lymphomas where one or more lines of treatment have failed or have been rendered inadequate or insufficient to render a response |
| Launched - 2022 | Bristol-Myers Squibb | Multiple myeloma | Treatment of adult patients who have received at least two prior therapies, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody, and have experienced disease progression or relapse after the last therapy |
| Launched - 2021 | 2seventy bio | Multiple myeloma | Treatment of adult patients with relapsed or refractory multiple myeloma after two or more prior lines of therapy including an immunomodulatory agent (IMiD), a proteasome inhibitor (PI), and an anti-CD38 monoclonal antibody |
| Launched - 2022 | Bristol-Myers Squibb | B-cell leukemia | Second-line therapy of recurrent or refractory large B-cell lymphoma |
| Launched - 2024 | Bristol-Myers Squibb | Chronic lymphocytic leukemia | Treatment of adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) who have received at least two prior lines of therapy, including a Bruton tyrosine kinase (BTK) inhibitor and a B-cell lymphoma 2 (BCL-2) inhibitor |
| Launched - 2021 | Bristol-Myers Squibb | Lymphoma, B-cell | Treatment of adult patients with relapsed or refractory high-grade B-cell lymphoma after two or more lines of systemic therapy; Treatment of adult patients with relapsed or refractory high-grade B-cell lymphoma who have refractory disease to first-line chemoimmunotherapy, relapse within 12 months of first-line chemoimmunotherapy including who are not eligible for hematopoietic stem cell transplantation (HSCT) due to comorbidities or age |
| Launched - 2024 | Bristol-Myers Squibb | Lymphoma, small lymphocytic | Treatment of adult patients with relapsed or refractory small lymphocytic lymphoma (SLL) who have received at least two prior lines of therapy, including a Bruton tyrosine kinase (BTK) inhibitor and a B-cell lymphoma 2 (BCL-2) inhibitor |
| Registered - 2023 | Bristol-Myers Squibb | Lymphoma, B-cell | In adult patients with diffuse large B-cell lymphoma (DLBCL), high grade B-cell lymphoma (HGBCL), primary mediastinal large B-cell lymphoma (PMBCL) and follicular lymphoma grade 3B (FL3B), who relapsed within 12 months from completion of, or are refractory to, first-line chemoimmunotherapy |
| Phase I | Seattle Children's Hospital | Acute lymphocytic leukemia | Treatment of children and young adults (aged 1 to 26) with relapsed CD19+ acute lymphoblastic leukemia, following cyclophosphamide administration |
| Registered - 2023 | Juventas Cell Therapy | B-cell acute lymphocytic leukemia | For adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia (r/r B-ALL) |
(Disclaimer: The information in this table is just for knowledge sharing and not for marketing or sales purposes.)
If you require more information about our anti-CD137 recombinant antibodies, please do not hesitate to contact us at your convenience. We are excited to embark on a collaborative journey and support your research endeavors with our specialized products and services.



