Anti-Human EGFR Recombinant Antibody (Cetuximab)
CAT#: TAB-003
Recombinant monoclonal antibody to Human EGFR. Cetuximab (Erbitux) is an epidermal growth factor receptor (EGFR) inhibitor used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.























Specifications
- Immunogen
- CHO cells transfected with a plasmid bearing a truncated form of EGFR cDNA
- Host Species
- Human
- Derivation
- Chimeric (mouse/human)
- Type
- IgG1 - kappa
- Specificity
- Tested positive against native human antigen
- Species Reactivity
- Human
- Applications
- IF, IP, Neut, FuncS, ELISA, FC, ICC, WB, Block, Activ, Inhib
- Trade name
- erbitux
- CAS
- 205923-56-4
- Generic Name
- Cetuximab
- Biological Half-Life
- 114 hrs
- ATC Code
- L01XC06
- DrugBank
- DB00002
- UNII
- PQX0D8J21J
- ChEMBL
- CHEMBL1201577
- MW
- 145,781.6 g/mol
- Related Disease
- Colorectal cancers, metastatic (EGFR positive)
Product Property
- Purity
- >95.0% as determined by analysis by SDS-PAGE.
- Storage
- Store the antibody (in aliquots) at -20°C. Avoid repeated freezing and thawing of samples.
Applications
- Application Notes
- The EGFR antibody has been reported in applications of IF, IP, Neut, FuncS, ELISA, FC, ICC, WB, Block, Activ, Inhib.
Target
Customer Review
There are currently no Customer reviews or questions for TAB-003. Click the button above to contact us or submit your feedback about this product.


Q&As
-
Is TAB-003 suitable for functional studies and other common assays?
A: Yes, TAB-003 is validated for functional studies (FuncS), making it ideal for cancer cell models. It is a chimeric (Human-Mouse) IgG1 and also has broad validation for other key applications, including Western Blot (WB), Flow Cytometry (FC), and ELISA, making it a very versatile reagent.
-
Can I use this antibody for imaging human EGFR, and what is its composition?
A: Absolutely. This antibody is a reliable choice for imaging, as it's confirmed to work consistently for Immunofluorescence (IF) on cells. It is a chimeric (Human-Mouse) IgG1 antibody that is recombinantly produced in a CHO expression system, ensuring high consistency.
View the frequently asked questions answered by Creative Biolabs Support.
Cite This Product
To accurately reference this product in your publication, please use the following citation information:
(Creative Biolabs Cat# TAB-003, RRID: AB_3111731)
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Biosimilar Overview
Please refer to Cetuximab Overview to learn more about the mechanism of action, clinical projects, and approved drugs of Cetuximab.
Related Diseases
Related Signaling Pathways
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Protocol & Troubleshooting
We have outlined the assay protocols, covering reagents, solutions, procedures, and troubleshooting tips for common issues in order to better assist clients in conducting experiments with our products. View the full list of Protocol & Troubleshooting.
See other products for "Cetuximab"
Afuco™ Anti-EGFR ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-003)This product is an ADCC enhanced antibody produced by our Afuco™ platform. Recombinant monoclonal antibody to Human EGFR. It is an epidermal growth factor receptor (EGFR) inhibitor used for the treatment of metastatic colorectal cancer and head and neck cancer. It is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.
See other products for "EGFR"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-750 | Anti-EGFR/HER1 Recombinant Antibody (TAB-750) | Neut, ELISA, IF, IP, FuncS, FC, WB | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1078z | Mouse Anti-EGFR Recombinant Antibody (clone 42C11) | WB, ELISA, FC, IF, IHC, FuncS | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NABG-056 | Recombinant Anti-Mouse Egfr VHH Single Domain Antibody | ELISA, IHC, FC, FuncS | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-H35 | Anti-Human EGFR Recombinant Antibody (Futuximab) | IF, WB, Inhib | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-020 | Anti-Human EGFR Recombinant Antibody (Panitumumab) | ELISA, IP, FC, FuncS, Neut, IF, ICC | IgG2 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-165 | Anti-Human EGFR Recombinant Antibody (Matuzumab) | Neut, ELISA, IF, IP, FuncS, FC, ICC | IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-710 | Anti-EGFR Recombinant Antibody (Nimotuzumab) | ELISA, IP, FC, FuncS, Neut, IF, IHC | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-040 | Anti-Human EGFR Recombinant Antibody (TAB-040) | ELISA, FC, IP, FuncS, IF, Neut, ICC | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-119 | Anti-Human EGFR Recombinant Antibody (TAB-119) | FC, IP, ELISA, Neut, FuncS, IF, WB | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-753 | Anti-EGFR Recombinant Antibody (Imgatuzumab) | Neut, ELISA, IF, IP, FuncS, FC, WB | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-H49 | Anti-Human EGFR Recombinant Antibody (Modotuximab) | FuncS, IF, Neut, ELISA, FC, IP, IHC | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-228CL | Anti-Human EGFR Recombinant Antibody (ABT-806) | WB, IHC | Antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-0242MC | Rabbit Anti-Human EGFR (phospho Y1092) Antibody | IHC, WB |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-0243MC | Rabbit Anti-Human EGFR (phospho Y1068) Antibody | IHC, WB |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-080 | Human Anti-EGFR Recombinant Antibody (PABL-080) | ELISA, WB, FuncS | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-080 | Human Anti-EGFR Recombinant Antibody; scFv Fragment (PSBL-080) | ELISA, WB, FuncS | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-080 | Human Anti-EGFR Recombinant Antibody; Fab Fragment (PFBL-080) | ELISA, WB, FuncS | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PNBL-016 | Recombinant Anti-Human EGFR VHH Single Domain Antibody (PNBL-016) | WB, ELISA | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PNBL-017 | Recombinant Anti-Human EGFR VHH Single Domain Antibody (PNBL-017) | FuncS, ELISA, IF | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PNBL-018 | Recombinant Anti-Human EGFR VHH Single Domain Antibody (PNBL-018) | FuncS, SPR | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABZ-039 | Mouse Anti-EGFR Recombinant Antibody (clone mAb528) | FC | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBZ-039 | Mouse Anti-EGFR Recombinant Antibody (clone mAb528); Fab Fragment | FC | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBW-039 | Human Anti-EGFR Recombinant Antibody Fab Fragment (PFBW-039) | FuncS | Chimeric (mouse/human) Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBC-040 | Human Anti-EGFR Recombinant Antibody (clone MR1); Fab Fragment | Block | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-459 | Human Anti-EGFR Recombinant Antibody (clone C225); Fab Fragment | FC | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBW-171 | Mouse Anti-EGFR Recombinant Antibody; Fab Fragment (PFBW-171) | WB | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBZ-039 | Mouse Anti-EGFR Recombinant Antibody (clone mAb528); scFv Fragment | FC | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBW-039 | Mouse Anti-EGFR Recombinant Antibody scFv Fragment (PSBW-039) | Block | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBC-040 | Human Anti-EGFR Recombinant Antibody (clone MR1); scFv Fragment | Block | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0225CL | Human Anti-EGFR Recombinant Antibody (TAB-0225CL) | Block, Inhib, FuncS, Apop, In vivo | Chimeric (Mouse/Human) IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0564CL | Mouse Anti-EGFR Recombinant Antibody (TAB-0564CL) | ELISA | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0565CL | Mouse Anti-EGFR Recombinant Antibody (TAB-0565CL) | ELISA | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0225CL-S(P) | Human Anti-EGFR Recombinant Antibody; scFv Fragment (TAB-0225CL-S(P)) | Block, Inhib, FuncS, Apop, In vivo | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0564CL-S(P) | Mouse Anti-EGFR Recombinant Antibody; scFv Fragment (TAB-0564CL-S(P)) | ELISA | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0565CL-S(P) | Mouse Anti-EGFR Recombinant Antibody; scFv Fragment (TAB-0565CL-S(P)) | ELISA | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0564CL-F(E) | Mouse Anti-EGFR Recombinant Antibody; Fab Fragment (TAB-0564CL-F(E)) | ELISA | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-270MZ | Human Anti-EGFR Recombinant Antibody (TAB-270MZ) | ELISA | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-274MZ | Human Anti-EGFR Recombinant Antibody (TAB-274MZ) | FC | Humanized IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-278MZ | Human Anti-EGFR Recombinant Antibody (TAB-278MZ) | Cyt, ELISA, Inhib | Human IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-015MZ-VHH | Anti-Human EGFR Recombinant Antibody (TAB-015MZ-VHH) | sELISA | Single domain antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-055LC | Recombinant Anti-Human EGFR Antibody (Fc glycosylation/High-mannose glycosylated) | ELISA | Chimeric antibody (mouse/human) |
| Gly-055LC-1 | Recombinant Anti-Human EGFR Antibody (Fc glycosylation/High-mannose glycosylated) | ELISA | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-144LC | Recombinant Anti-Human EGFR Antibody (Fc glycosylation) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-167LC | Recombinant Anti-Human EGFR Antibody (Non-glycosylated) | ELISA | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| BRD-0183MZ | Chicken Anti-EGFR Polyclonal IgY | WB | Chicken antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MHC-LC773 | A*0201/Human EGFR (YLNTVQPTCV) MHC Tetramer | FCM |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-722CQ | Rabbit Anti-EGFR Recombinant Antibody (clone CBL1011) | Neut | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-723CQ | Mouse Anti-EGFR Recombinant Antibody (clone CBL931) | WB, IP, IHC, ICC, Neut | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-724CQ | Rabbit Anti-EGFR Recombinant Antibody (NEUT-724CQ) | IF, FC, WB, IP, Neut | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1101 | Hi-Affi™ Rabbit Anti-EGFR Recombinant Antibody (clone DS1101AB) | IHC-P | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-4520 | Hi-Affi™ Rabbit Anti-EGFR Recombinant Antibody (clone TH28DS) | IF, ICC, FC | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-4570 | Hi-Affi™ Rabbit Anti-EGFR Recombinant Antibody (clone TH82DS) | ELISA | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-4571 | Hi-Affi™ Rabbit Anti-EGFR Recombinant Antibody (clone TH83DS) | WB, IF, ICC, FC | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-4675 | Hi-Affi™ Rabbit Anti-EGFR Recombinant Antibody (clone TH189DS) | WB, IF, ICC, FC | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MHC-LC4545 | PE-DQB1*03:02/Human EGFR (SRALEEKKGNYVVTHG) MHC Tetramer | FCM |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-165 | Afuco™ Anti-EGFR ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-165) | Neut, ELISA, IF, IP, FuncS, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-464CQ | Afuco™ Anti-EGFR ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-464CQ) | ELISA, IHC, FC, IP, IF, FuncS | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-003 | Afuco™ Anti-EGFR ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-003) | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-040 | Afuco™ Anti-EGFR ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-040) | ELISA, FC, IP, FuncS, IF, Neut | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-119 | Afuco™ Anti-EGFR ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-119) | FC, IP, ELISA, Neut, FuncS, IF | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0424-XY84 | AbPlus™ Anti-EGFR Magnetic Beads (pSEX81-6) | IP, Protein Purification |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0924-YC32 | Mouse Anti-EGFR Recombinant Antibody (VS-0924-YC32) - Cancer Stem Cell Marker | IHC, WB | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0924-YC35 | Rabbit Anti-EGFR Antibody (VS-0924-YC35) - Cancer Stem Cell Marker | IHC, WB, IF | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1024-XY177 | Mouse Anti-NHP EGFR Recombinant Antibody (clone 225) | IF, IP | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0125-FY28 | Human Anti-EGFR (clone ABT-806) scFv-Fc Chimera | FC, Cyt | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY102 | CytoStream™ Mouse Anti-EGFR Recombinant Antibody (VS-0225-XY102) | FC | Mouse IgG1, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-XY735 | Anti-EGFR Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0425-YC340 | Recombinant Anti-EGFR Vesicular Antibody, EV Displayed (VS-0425-YC340) | ELISA, FC, Neut, Cell-uptake |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY2183 | Anti-Mouse EGFR Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC65 | Recombinant Anti-EGFR (AA 269-278 x AA 526-535) Biparatopic Antibody, Tandem scFv (Clone Pep 2 x Clone Pep 3) | FC | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC66 | Recombinant Anti-EGFR (AA 582-591 x AA 606-614) Biparatopic Antibody, Tandem scFv (Clone Pep 4 x Clone Pep 1) | FC | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC68 | Recombinant Anti-EGFR (AA 526-535 x AA 600-605) Biparatopic Antibody, Tandem scFv (Clone Pep 3 x Clone Pep 5) | FC | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC213 | Recombinant Anti-EGFR (Domain II x Domain III) Biparatopic Antibody, Tandem scFv | ELISA, FC, IF, IHC, IP | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY2182 | Anti-Human EGFR Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC110 | SmartAb™ Recombinant Anti-EGFR pH-dependent Antibody (VS-0825-YC110) | Neut, ELISA, IF, IP, FC, WB | Human IgG1 kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1025-YC4 | Anti-EGFR Antibody Prodrug, Protease Activated (clone 528) | ISZ, Cyt, FuncS |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1025-YC5 | Anti-EGFR Antibody Prodrug, Protease Activated (Cetuximab) | ISZ, Cyt, FuncS |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1025-YC6 | Anti-EGFR Antibody Prodrug, Protease Activated (Panitumumab) | ISZ, Cyt, FuncS |
Popular Products

Application: ELISA, IHC

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: FuncS, IF, Neut, ELISA, FC, IP, IHC

Application: Block, Cyt, FuncS, Inhib

Application: Neut, Inhib, FC, ELISA

Application: Neut, ELISA, FuncS

Application: ELISA, IHC, FC, IP, IF, FuncS

Application: ELISA

Application: Neut, ELISA, WB, SPR, IF, EM

Application: FC

Application: ELISA, FC, FuncS

Application: WB, IHC-Fr, IHC-P, ICC, IF
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.


























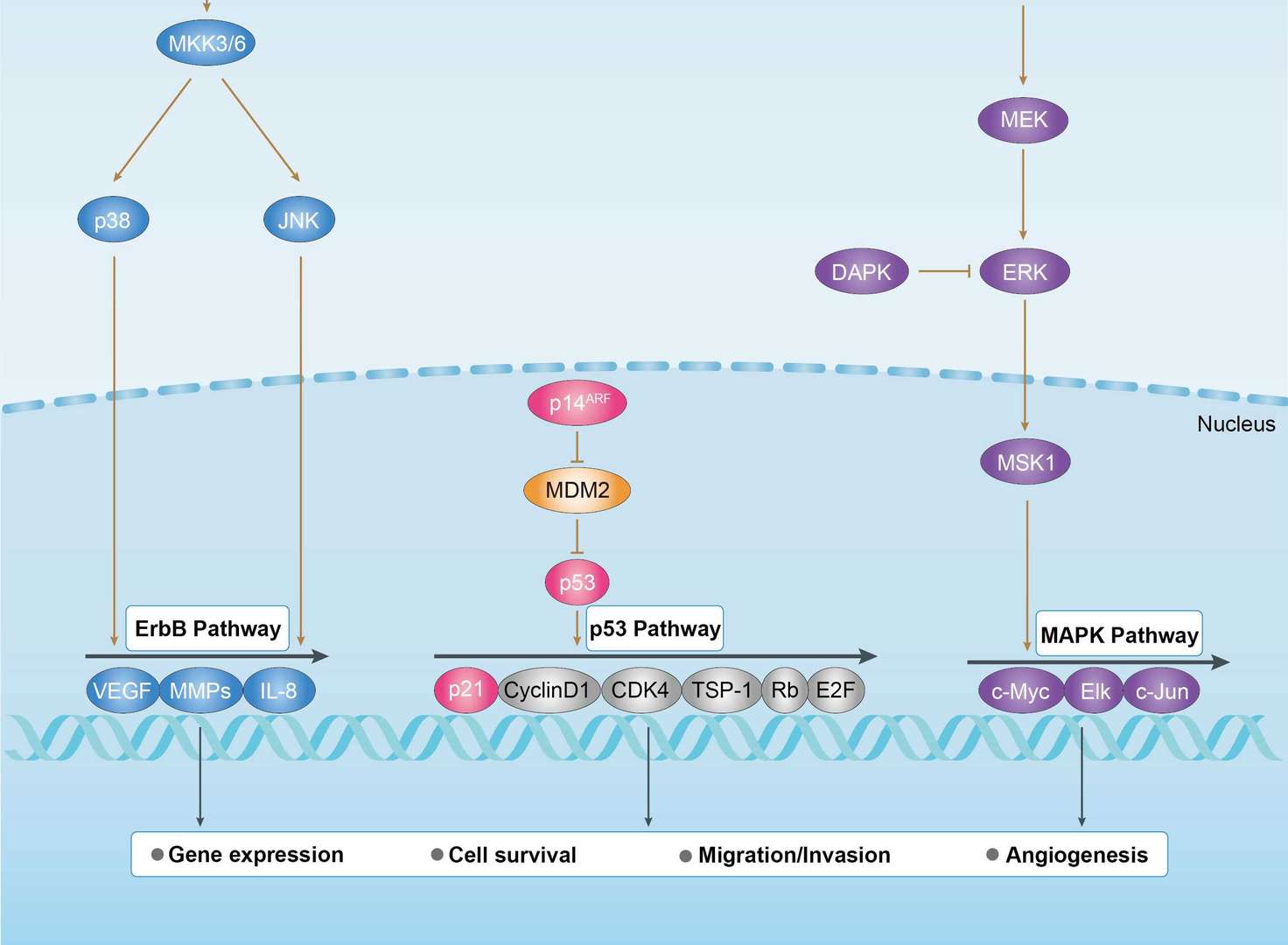 Bladder Cancer
Bladder Cancer
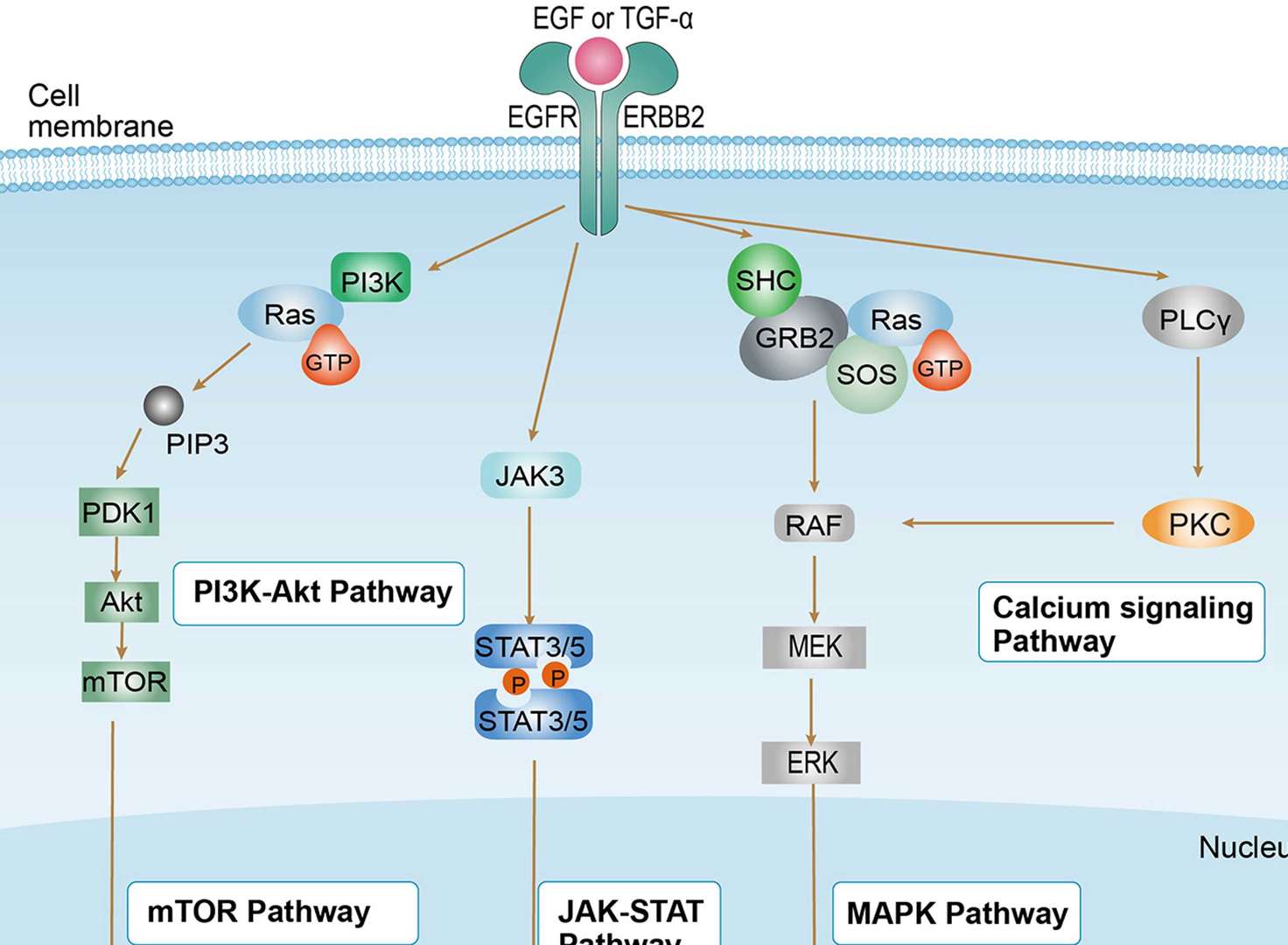 Non-small Cell Lung Cancer
Non-small Cell Lung Cancer
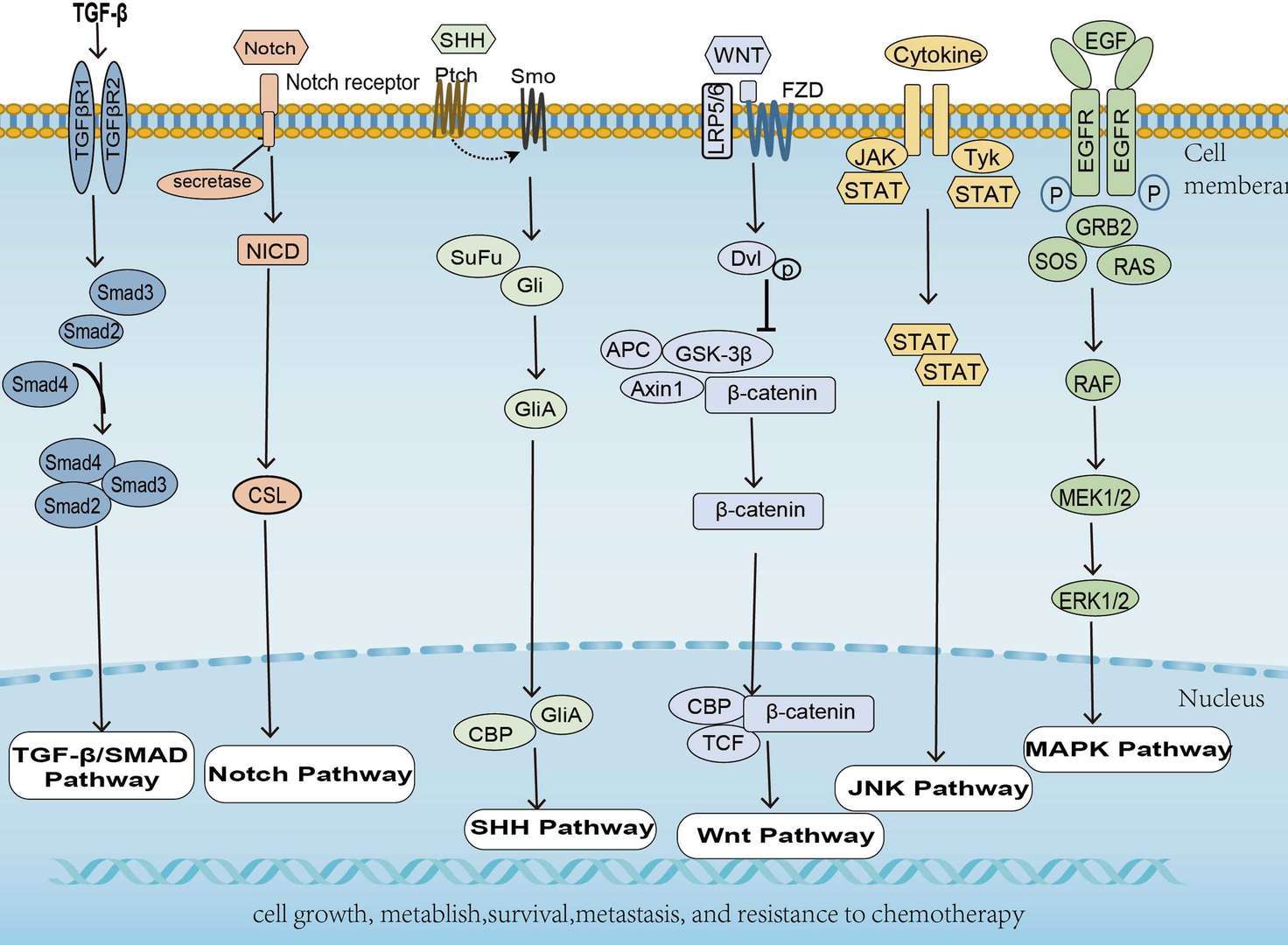 Pancreatic Cancer
Pancreatic Cancer
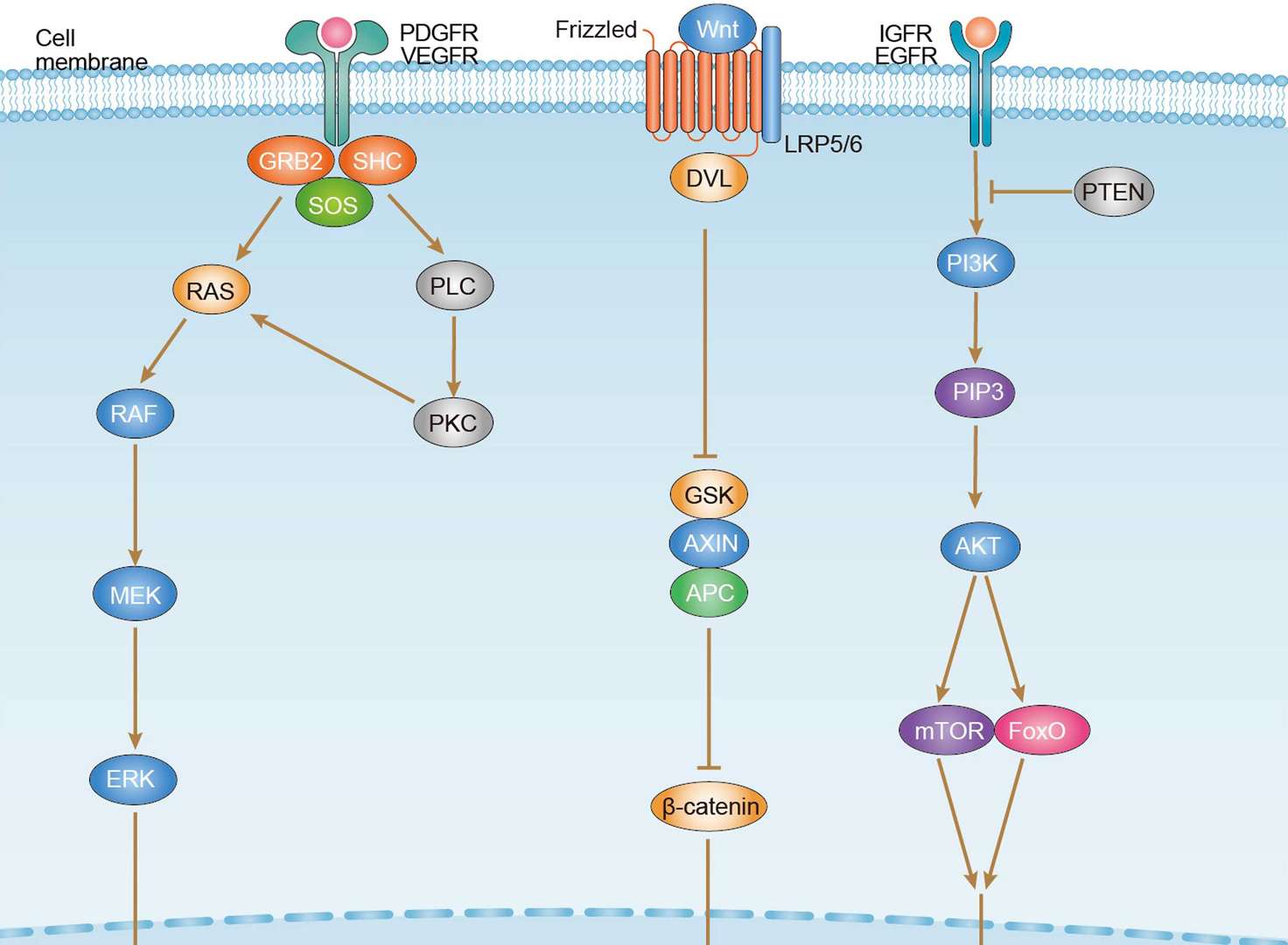 Hepatocellular Carcinoma
Hepatocellular Carcinoma
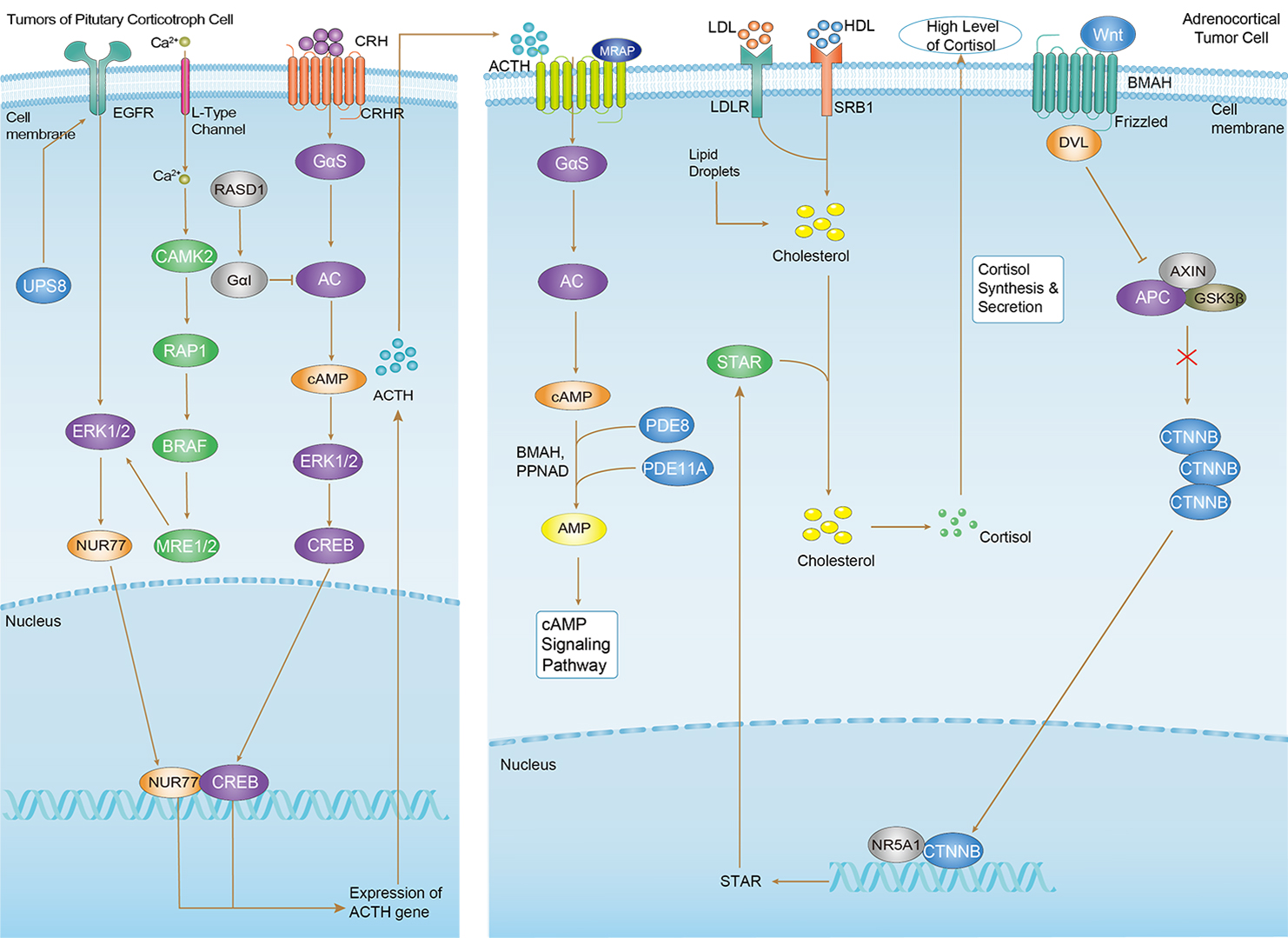 Cushing Syndrome
Cushing Syndrome
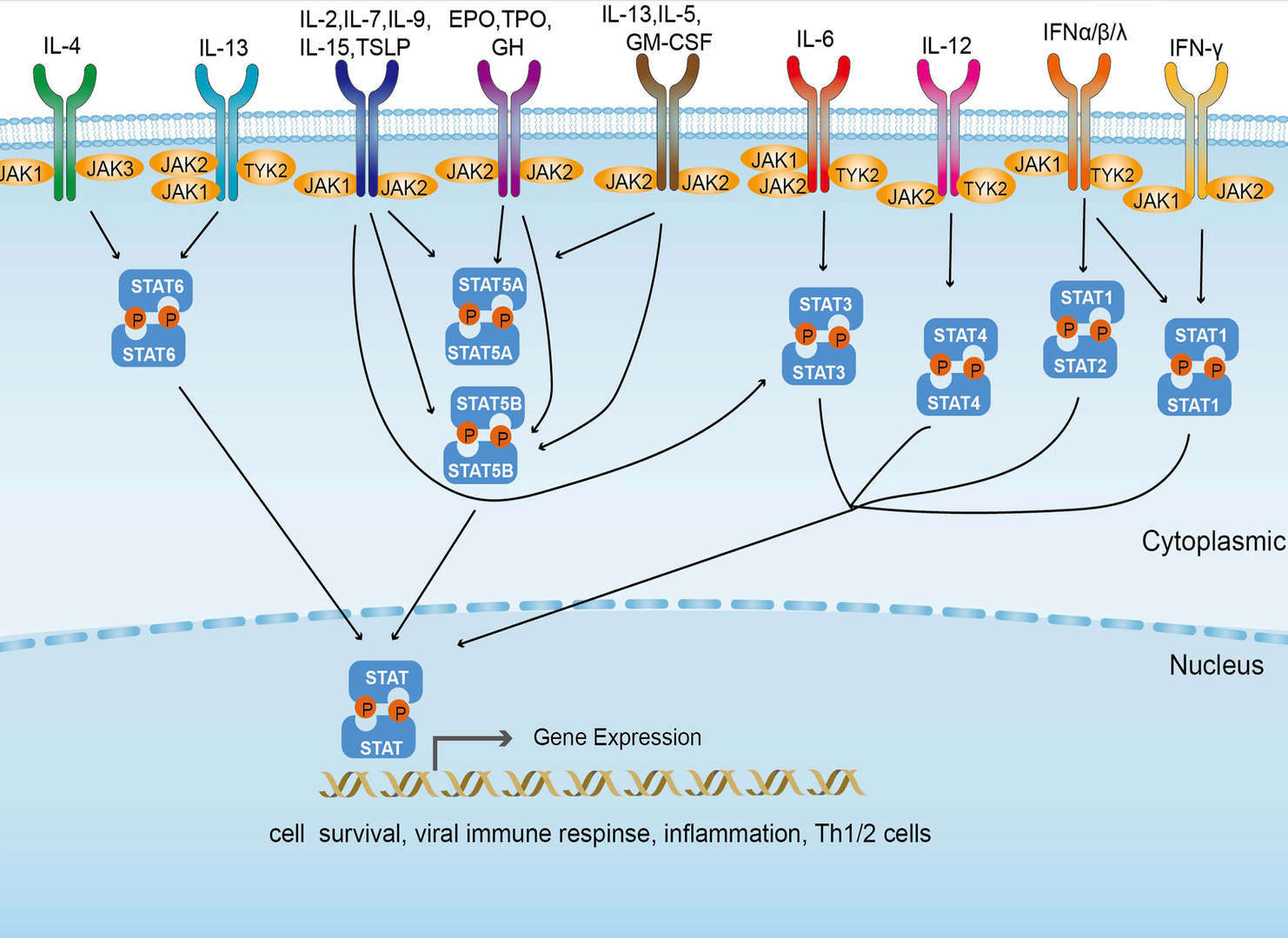 JAK-STAT Signaling Pathway
JAK-STAT Signaling Pathway












