Anti-Human IGF1 Receptor Recombinant Antibody (Cixutumumab)
CAT#: TAB-078
Recombinant monoclonal antibody to IGF1 Receptor. Cixutumumab is a monoclonal antibody for the treatment of solid tumors.
It is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor (IGF-1R) with potential antineoplastic activity. Cixutumumab selectively binds to membrane-bound IGF-1R, thereby preventing the binding of the natural ligand IGF-1 and the subsequent activation of PI3K/AKT signaling pathway. Downregulation of the PI3K/AKT survival pathway may result in the induction of cancer cell apoptosis and may decrease cancer cellular proliferation. IGF-1R, a receptor tyrosine kinase of the insulin receptor superfamily overexpressed by many cancer cell types, stimulates cell proliferation, enables oncogenic transformation, and suppresses apoptosis; IGF-1R signaling has been implicated in tumorigenesis and metastasis.


















Specifications
- Immunogen
- The details of the immunogen for this antibody are not available.
- Host Species
- Human
- Derivation
- Human
- Type
- IgG1 - lambda
- Specificity
- Tested positive against native human antigen.
- Species Reactivity
- Human
- Applications
- IP, Neut, FuncS, ELISA, FC, WB, IHC, Stim, IF, Block
- CAS
- 947687-12-9
- Generic Name
- Cixutumumab
- UNII
- 2285XW22DR
- MW
- 146.3 kDa
- Related Disease
- Non-small cell lung cancers (NSCLC)
Product Property
- Purity
- >95.0%, determined by analysis by RP-HPLC & analysis by SDS-PAGE.
- Storage
- Store at 4°C for up to 3 months. For longer term storage aliquot into small volumes and store at -20°C.
Applications
- Application Notes
- The IGF1R antibody has been reported in applications of IP, Neut, FuncS, ELISA, FC, WB, IHC, Stim, IF, Block.
Target
Customer Review
There are currently no Customer reviews or questions for TAB-078. Click the button above to contact us or submit your feedback about this product.


Q&As
-
What is the mechanism of action for this antibody in a research context?
A: Cixutumumab is a fully human IgG1 monoclonal antibody that selectively binds to membrane-bound IGF-1R. In research models, this prevents the binding of the natural ligand IGF-1, thereby inhibiting the activation of the downstream PI3K/AKT signaling pathway and potentially inducing apoptosis in cancer cells.
-
What applications has this antibody been verified for?
A: This antibody has been extensively reported for use in a wide range of applications, including Immunoprecipitation (IP), Neutralization (Neut), Functional Assays (FuncS), ELISA, Flow Cytometry (FC), Western Blot (WB), Immunohistochemistry (IHC), Stimulation (Stim), Immunofluorescence (IF), and Blocking studies.
View the frequently asked questions answered by Creative Biolabs Support.
Citations
-
Solomon, Viswas Raja, et al. "111In-and 225Ac-labeled cixutumumab for imaging and α-particle radiotherapy of IGF-1R positive triple-negative breast cancer." Molecular Pharmaceutics 16.12 (2019): 4807-4816. https://doi.org/10.1021/acs.molpharmaceut.9b00542This research focuses on developing a theranostic approach for targeting IGF-1R positive triple-negative breast cancer (TNBC) using radiolabeled cixutumumab. The study describes the conjugation of cixutumumab, a humanized monoclonal antibody that binds to IGF-1R with low nanomolar affinity, with p-SCN-Bn-DOTA chelator and subsequent radiolabeling with either 111In for imaging or 225Ac for alpha-particle radiotherapy. The researchers demonstrated that the radiolabeled cixutumumab maintained its binding affinity to IGF-1R and was effectively internalized in TNBC cells. In vitro studies showed that 225Ac-cixutumumab was approximately 5000-fold more cytotoxic than unlabeled cixutumumab. In vivo studies using SUM149PT xenograft models revealed that low specific activity 225Ac-cixutumumab (0.15 kBq/μg) showed superior therapeutic efficacy compared to high specific activity preparation, leading to complete tumor remission in some cases and significantly improved survival rates.
Creative Biolabs provided the monoclonal antibody cixutumumab (IMC-A12) that served as the foundation for this research. This antibody, with its high specificity for IGF-1R, was crucial for the development of the radioimmunoconjugates that showed promising results in both imaging and therapeutic applications. The availability of this high-quality antibody enabled the researchers to successfully create a theranostic pair that could potentially address the clinical limitations of previous anti-IGF-1R treatments, offering a new strategy for treating TNBC patients with IGF-1R overexpression through targeted alpha-particle radiotherapy.
Cite This Product
To accurately reference this product in your publication, please use the following citation information:
(Creative Biolabs Cat# TAB-078, RRID: AB_3111795)
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Biosimilar Overview
Please refer to Cixutumumab Overview to learn more about the mechanism of action, clinical projects, and approved drugs of Cixutumumab.
Related Signaling Pathways
Related Diseases
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Protocol & Troubleshooting
We have outlined the assay protocols, covering reagents, solutions, procedures, and troubleshooting tips for common issues in order to better assist clients in conducting experiments with our products. View the full list of Protocol & Troubleshooting.
See other products for "Cixutumumab"
DrugMonitor™ Anti-Cixutumumab Antibody (VS-1224-YC284)Cixutumumab has been evaluated in clinical trials for treating lung cancer, malignant neoplasms, leukemia, mast-cell neoplasms, non-small-cell lung carcinoma, and adenocarcinoma of the prostate, known for its high affinity for the insulin-like growth factor-I receptor (IGF-IR) overexpressed in various tumors. The DrugMonitor™ Anti-Cixutumumab Antibody (VS-1224-YC284) is an anti-drug antibody (ADA) against Cixutumumab. This drug-based antibody is raised in mice immunized with the Cixutumumab. The anti-Cixutumumab antibody may be used in ELISA, pharmacokinetics (PK), and pharmacodynamics (PD) analyses, or serves as a reference standard in ADA assays. It also is an excellent tool for therapeutic drug monitoring, allowing to evaluate the drug efficacy and determine the drug concentration of the Cixutumumab in samples.
Afuco™ Anti-IGF1R ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-078)This product is an ADCC enhanced antibody produced by our Afuco™ platform. Recombinant monoclonal antibody to IGF1 Receptor. It is a monoclonal antibody for the treatment of solid tumors.
It is a fully human IgG1 monoclonal antibody directed against the human insulin-like growth factor-1 receptor (IGF-1R) with potential antineoplastic activity.
See other products for "IGF1R"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-1121z | Mouse Anti-IGF1R Recombinant Antibody (clone 19H8) | WB, ELISA, IHC | Mouse IgG2b |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NABL-091 | Recombinant Anti-human IGF1R VHH Single Domain Antibody | WB, ELISA, IHC, FC, FuncS | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-199 | Human Anti-IGF1R Recombinant Antibody (TAB-199) | FC, IP, ELISA, Neut, FuncS, IF, ICC | Human IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-232 | Anti-Human IGF1 Receptor Recombinant Antibody (Figitumumab) | WB, IF, IP, Neut, FuncS, ELISA, FC | IgG2 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-736 | Anti-IGF1R Recombinant Antibody (TAB-736) | FC, IP, ELISA, Neut, FuncS, IF, ICC | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-209 | Anti-Human IGF1 Receptor Recombinant Antibody (TAB-209) | FuncS, IF, Neut, ELISA, FC, IP, ICC | IgG1 - kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-L024E | IGF1-PE immunotoxin | Cytotoxicity assay, Functional assay |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-L024D | IGF1-DT immunotoxin | Cytotoxicity assay, Functional assay |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-386CL | Humanized Anti-IGF1R Recombinant Antibody (TAB-386CL) | FuncS | Humanized IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-371CL | Afuco™ Anti-Human IGF1R Antibody, ADCC Enhanced (AFC-371CL) | ELISA | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-596 | Mouse Anti-IGF1R Recombinant Antibody (clone mAb 83-14) | WB, ELISA, FuncS | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABL-597 | Mouse Anti-IGF1R Recombinant Antibody (clone mAb 83-7) | WB, ELISA, FuncS | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-591 | Mouse Anti-IGF1R Recombinant Antibody (clone mAb 83-14); Fab Fragment | WB, ELISA, FuncS | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-592 | Mouse Anti-IGF1R Recombinant Antibody (clone mAb 83-7); Fab Fragment | WB, ELISA, FuncS | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-591 | Mouse Anti-IGF1R Recombinant Antibody (clone mAb 83-14); scFv Fragment | WB, ELISA, FuncS | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PSBL-592 | Mouse Anti-IGF1R Recombinant Antibody (clone mAb 83-7); scFv Fragment | WB, ELISA, FuncS | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-009ZJ | Mouse Anti-IGF1R Recombinant Antibody (TAB-009ZJ) | ELISA, FC | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-010ZJ | Anti-Human IGF-1R Recombinant Antibody (6E11) | ELISA, Neut, FC, IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-011ZJ | Mouse Anti-IGF1R Recombinant Antibody (TAB-011ZJ) | ELISA, FC, WB, Inhib | Mouse IgG2, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-012ZJ | Mouse Anti-IGF1R Recombinant Antibody (TAB-012ZJ) | ELISA, FC, WB, Inhib | Mouse IgG2, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-013ZJ | Anti-Human IGF-1R Recombinant Antibody (1H7) | WB, Inhib |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-035ZJ | Human Anti-IGF1R Recombinant Antibody (TAB-035ZJ) | ELISA, FC | Humanized IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-036ZJ | Anti-Human IGF-1R Recombinant Antibody (H0L0 IgGIm(AA)) | ELISA, Neut, FC, IHC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-037ZJ | Anti-Human IGF-1R Recombinant Antibody (H1L0 IgGIm(AA)) | ELISA, Neut, FC, IHC | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-052ZJ | Anti-Human IGF-1R Recombinant Antibody (TAB-052ZJ) | IHC, ELISA, FC, IF, IP | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-053ZJ | Anti-Human IGF-1R Recombinant Antibody (IR3) | ELISA, FC, WB | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-084ZJ | Human Anti-IGF1R Recombinant Antibody (TAB-084ZJ) | WB, Inhib, ELISA, FC | Chimeric (mouse/human) IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-085ZJ | Anti-Human IGF-1R Recombinant Antibody (6E11c) | ELISA, FC, IHC, Inhib | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-086ZJ | Human Anti-IGF1R Recombinant Antibody (TAB-086ZJ) | ELISA, FC, Inhib, IP | Chimeric (mouse/human) IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-087ZJ | Anti-Human IGF-1R Recombinant Antibody (ch7C2) | ELISA | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-093ZJ | Anti-Human IGF-1R Recombinant Antibody (ch9E11) | ELISA | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-096ZJ | Anti-Human IGF-1R Single Domain Antibody (TAB-096ZJ), Research Grade Biosimilar | ELISA, IHC, IF, WB | Single domain antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOB-2249MZ | Recombinant Mouse Anti-Human IGF1R Antibody (clone 3D9) | WB | Mouse antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| BRD-0103MZ | Chicken Anti-CD221 Polyclonal IgY | WB | Chicken antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1075CQ | Mouse Anti-IGF1R Recombinant Antibody (clone alphaIR3) | FC, IP, Neut, ICC, IF | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1076CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 33255.111) | ELISA, Neut, WB | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1077CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 1H7) | FC, Block, IHC, IP, WB | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1078CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 24-60) | Inhib, ICC, IF, IP, WB | Mouse IgG2a, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1079CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 17-69) | Inhib, IP | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1080CQ | Mouse Anti-IGF1R Recombinant Antibody (clone 24-57) | Inhib, IP | Mouse IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1081CQ | Mouse Anti-IGF1R Recombinant Antibody (clone CBL453) | WB, ELISA, Neut | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-1082CQ | Mouse Anti-IGF1R Recombinant Antibody (clone CBL080) | Neut, WB | Mouse IgG1 |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1755 | Rabbit Anti-IGF1R Recombinant Antibody (clone DS1755AB) | IP, ELISA | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-4684 | Rabbit Anti-IGF1R Recombinant Antibody (clone TH198DS) | WB, ELISA | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-4685 | Rabbit Anti-IGF1R Recombinant Antibody (clone TH199DS) | WB | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0084-YC | Human Anti-IGF1R Recombinant Antibody (HPAB-0084-YC) | ELISA, FC | Humanized IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0084-YC-S(P) | Human Anti-IGF1R Recombinant Antibody; scFv Fragment (HPAB-0084-YC-S(P)) | ELISA, FC | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0085-YC-S(P) | Human Anti-IGF1R Recombinant Antibody; scFv Fragment (HPAB-0085-YC-S(P)) | Block, FuncS, FC, ELISA | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0084-YC-F(E) | Human Anti-IGF1R Recombinant Antibody; Fab Fragment (HPAB-0084-YC-F(E)) | ELISA, FC | Humanized Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0085-YC-F(E) | Human Anti-IGF1R Recombinant Antibody; Fab Fragment (HPAB-0085-YC-F(E)) | Block, FuncS, FC, ELISA | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NS-041CN | Mouse Anti-IGF1R Recombinant Antibody (NS-041CN) | ELISA, WB, FC | Mouse IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NS-041CN-F(E) | Mouse Anti-IGF1R Recombinant Antibody; Fab Fragment (NS-041CN-F(E)) | ELISA, WB, FC | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NS-041CN-S(P) | Mouse Anti-IGF1R Recombinant Antibody; scFv Fragment (NS-041CN-S(P)) | ELISA, WB, FC | Mouse scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0210CQ-F(E) | Human Anti-IGF1R Recombinant Antibody (clone 15H12); Fab Fragment | ELISA, FC, Neut | Human Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-0210CQ-S(P) | Human Anti-IGF1R Recombinant Antibody (clone 15H12); scFv Fragment | ELISA, FC, Neut | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-199 | Afuco™ Anti-IGF1R Recombinant Antibody (AFC-TAB-199), ADCC Enhanced | FC, IP, ELISA, Neut, FuncS, IF | Human IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-209 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-209) | FuncS, IF, Neut, ELISA, FC, IP | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-736 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-736) | FC, IP, ELISA, Neut, FuncS, IF | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-078 | Afuco™ Anti-IGF1R ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-078) | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-1545-FY | Recombinant Llama Anti-IGF1R Single Domain Antibody (HPAB-1545-FY) | WB, ELISA | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-1546-FY | Recombinant Llama Anti-IGF1R Single Domain Antibody (HPAB-1546-FY) | WB, ELISA | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| HPAB-1547-FY | Recombinant Llama Anti-IGF1R Single Domain Antibody (HPAB-1547-FY) | WB, ELISA | Llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0424-XY145 | AbPlus™ Anti-IGF1R Magnetic Beads (9E11) | IP, Protein Purification |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0125-FY20 | Human Anti-IGF1R (clone 1H3) scFv-Fc Chimera | ELISA, FC, Neut, Inhib | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-FY85 | Human Anti-IGF1R (clone 7C10) scFv-Fc Chimera | ELISA, FC, Inhib | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0325-XY1101 | Anti-IGF1R Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0425-YC366 | Recombinant Anti-IGF1R Vesicular Antibody, EV Displayed (VS-0425-YC366) | ELISA, FC, Neut, Cell-uptake |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY3431 | Anti-Mouse IGF1R Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC108 | Recombinant Anti-IGF1R (AA 62-184 x AA 283-440) Biparatopic Antibody, Tandem scFv (Clone 4-52 x Clone 45742) | ELISA | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC107 | Recombinant Anti-IGF1R (AA 283-440 x AA 440-514) Biparatopic Antibody, Tandem scFv (Clone 45742 x Clone 24-55) | ELISA | Tandem scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY3430 | Anti-Human IGF1R Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY3432 | Anti-Rat IGF1R Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0825-YC167 | SmartAb™ Recombinant Anti-IGF1R pH-dependent Antibody (Clone DalotuzumAb) | ELISA, IHC, FC, IP, IF, WB | Human IgG1 kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-1025-YC73 | Anti-IGF1R Antibody Prodrug, Protease Activated (VS-1025-YC73) | ISZ, Cyt, FuncS |
Popular Products

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: FC, Cyt, Stim, PP, Agonist

Application: IF, IP, Neut, FuncS, ELISA, FC, ICC

Application: FC, IP, ELISA, Neut, FuncS, IF, WB

Application: IF, IP, Neut, FuncS, ELISA, FC, WB

Application: WB, Neut, ELISA, IF, IP, FuncS, FC

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: IHC, ELISA, FC, WB, ADCC, FuncS

Application: ELISA, FC, IF, WB

Application: WB, ELISA, FuncS, IB, FC, SPR, Apop

Application: ELISA, IHC, FC, IP, IF, Inhib

Application: ELISA, IHC, FC, IP, IF, BL

Application: IF, IP, Neut, FuncS, ELISA, FC
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.





















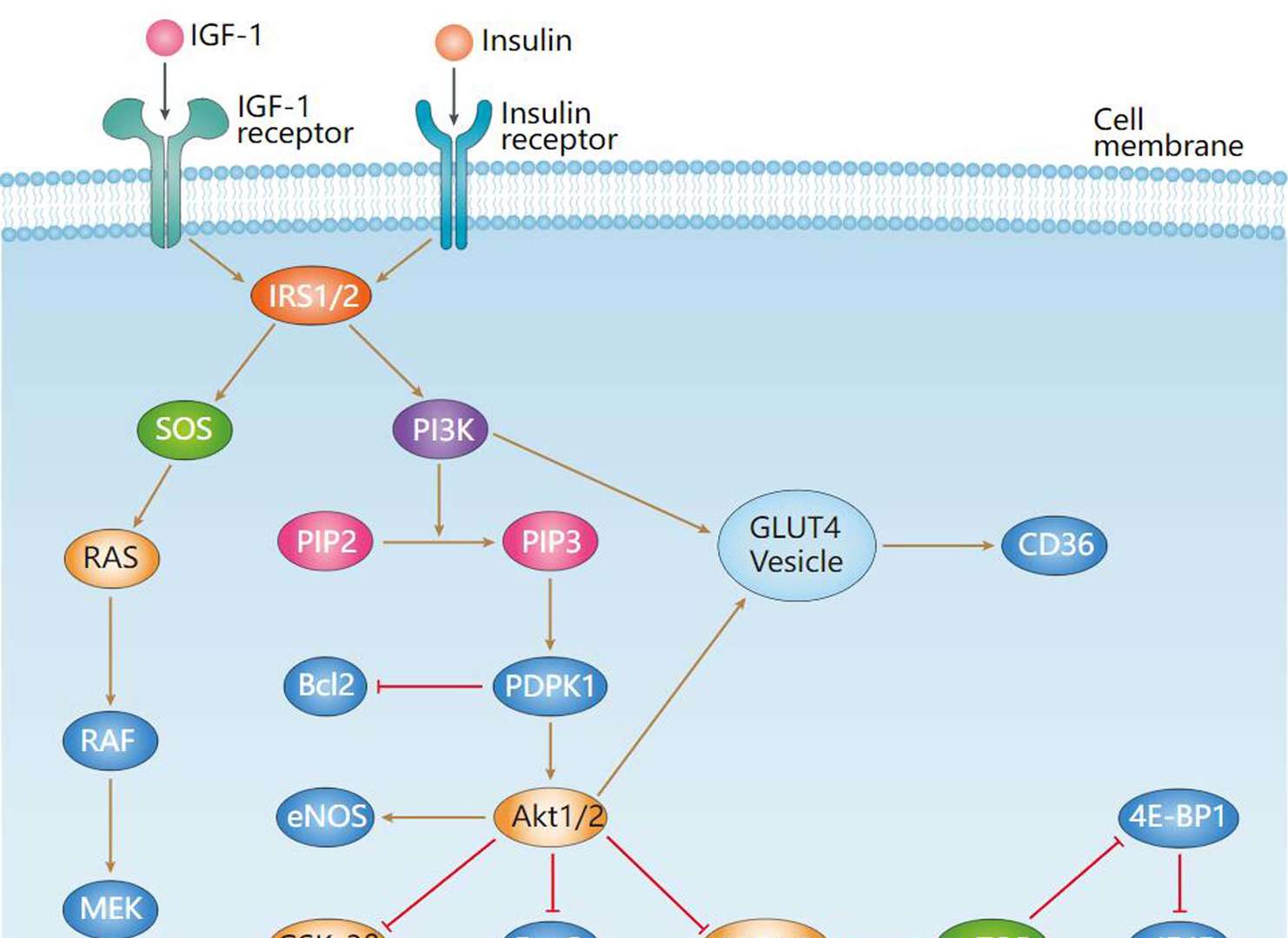 Insulin Signaling Pathway
Insulin Signaling Pathway
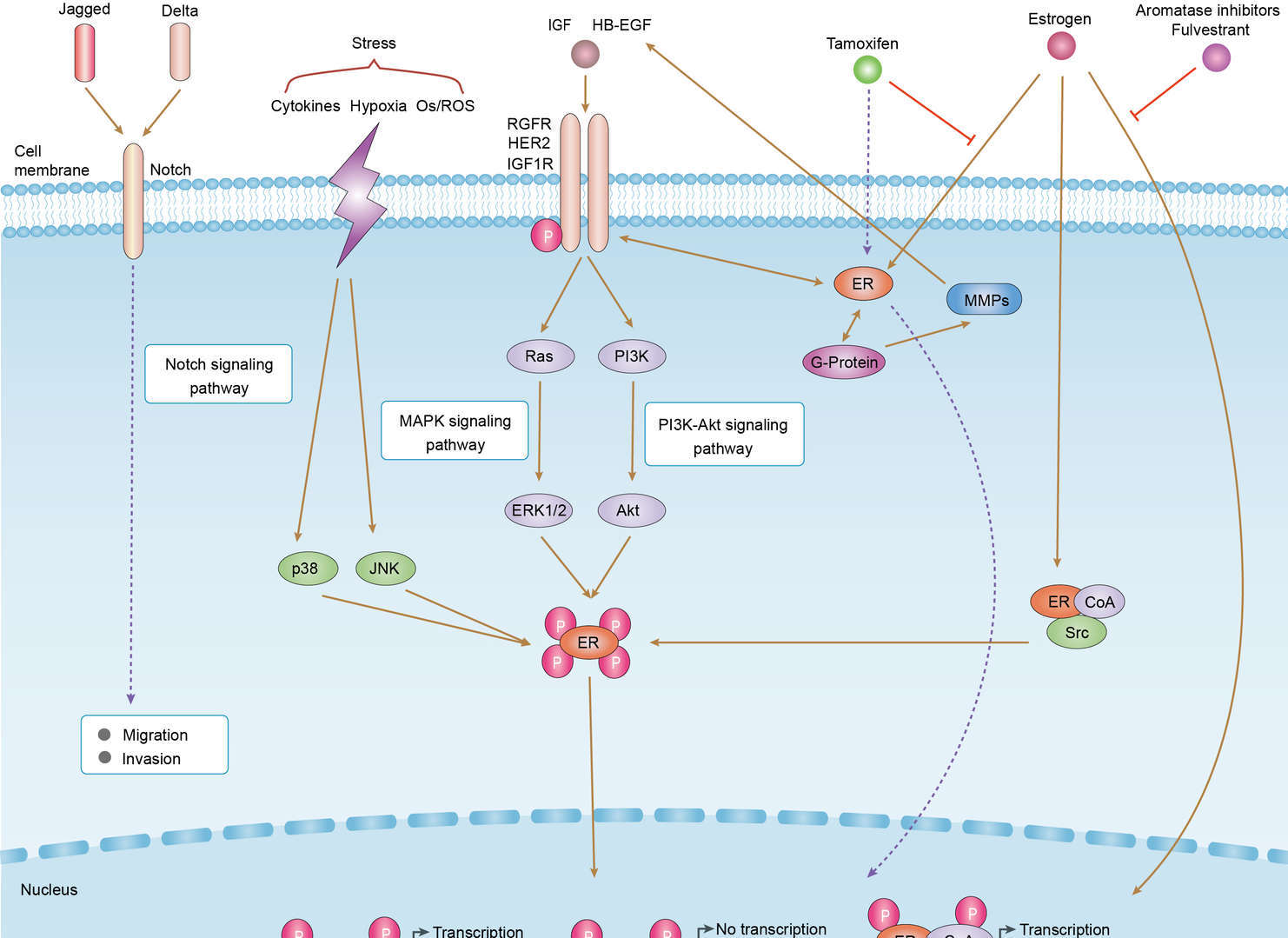 Endocrine Resistance
Endocrine Resistance
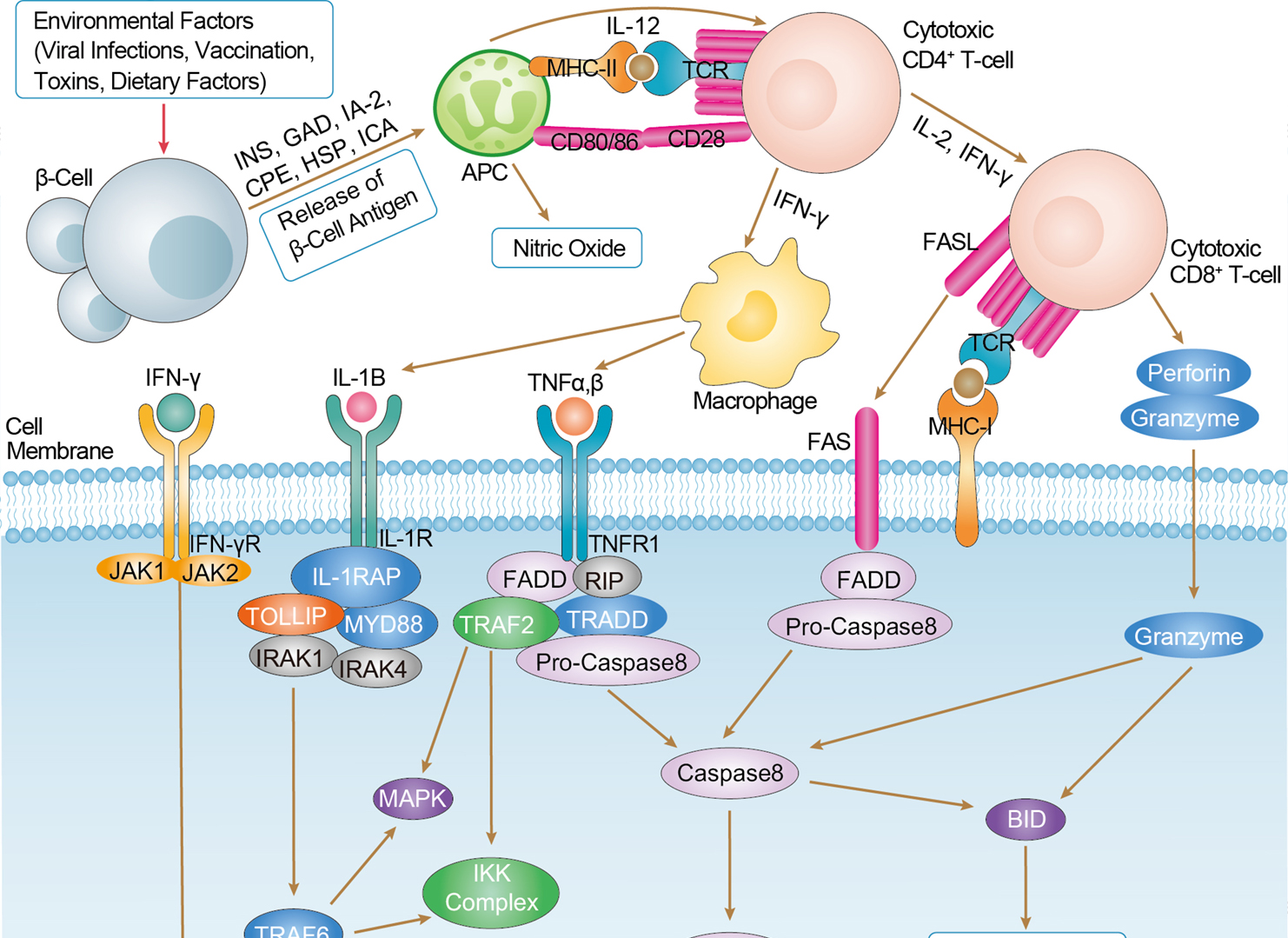 Type I Diabetes Mellitus
Type I Diabetes Mellitus











