Human Anti-ERBB2 Recombinant Antibody (clone FWP51)
CAT#: FAMAB-1130CQ
This product is a recombinant human antibody that recognizes ERBB2. The antibody was purified by affinity chromatography. It can be used in IP, FC, IF.










Specifications
- Host Species
- Human
- Type
- Human IgG1
- Specificity
- The extracellular domain of c-erbB-2
- Species Reactivity
- Human
- Clone
- FWP51
- Applications
- IP, FC, IF
Product Property
- Purity
- >95% as determined by SDS-PAGE
- Concentration
- Please refer to the vial label for the specific concentration.
- Storage
- Centrifuge briefly prior to opening vial. Store at +4°C short term (1-2 weeks). Aliquot and store at -20°C long term. Avoid repeated freeze/thaw cycles.
Target
- Alternative Names
- Erb-B2 Receptor Tyrosine Kinase 2; V-Erb-B2 Avian Erythroblastic Leukemia Viral Oncogene Homolog 2; Tyrosine Kinase-Type Cell Surface Receptor HER2; Neuro/Glioblastoma Derived Oncogene Homolog; Human Epidermal Growth Factor Receptor 2; Metastatic Lymph Node Gene 19 Protein; Proto-Oncogene C-ErbB-2; Proto-Oncogene Neu; EC 2.7.10.1; P185erbB2; MLN 19; HER2; NGL; NEU; V-Erb-B2 Avian Erythroblastic Leukemia Viral Oncogene Homolog 2 (Neuro/Glioblastoma Derived Oncogene Homolog)
- Gene ID
- 2064
- UniProt ID
- P04626
Customer Review
There are currently no Customer reviews or questions for FAMAB-1130CQ. Click the button above to contact us or submit your feedback about this product.
Submit Your Publication
Published with our product? Submit your paper and receive a 10% discount on your next order! Share your research to earn exclusive rewards.
Related Diseases
Related Signaling Pathways
Downloadable Resources
Download resources about recombinant antibody development and antibody engineering to boost your research.
Product Notes
This is a product of Creative Biolabs' Hi-Affi™ recombinant antibody portfolio, which has several benefits including:
• Increased sensitivity
• Confirmed specificity
• High repeatability
• Excellent batch-to-batch consistency
• Sustainable supply
• Animal-free production
See more details about Hi-Affi™ recombinant antibody benefits.
Datasheet
MSDS
COA
Certificate of Analysis LookupTo download a Certificate of Analysis, please enter a lot number in the search box below. Note: Certificate of Analysis not available for kit components.
Protocol & Troubleshooting
We have outlined the assay protocols, covering reagents, solutions, procedures, and troubleshooting tips for common issues in order to better assist clients in conducting experiments with our products. View the full list of Protocol & Troubleshooting.
See other products for "Clone FWP51"
- CAT
- Product Name
See other products for "ERBB2"
Select a product category from the dropdown menu below to view related products.
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NABG-059 | Recombinant Anti-Human ERBB2 VHH Single Domain Antibody | IHC, FC, CA, FuncS | Llama VHH |
| PNBL-066 | Recombinant Anti-HER2 VHH Single Domain Antibody (PNBL-066) | SPR | Llama VHH |
| PNBL-067 | Recombinant Anti-HER2 VHH Single Domain Antibody (PNBL-067) | SPR | Llama VHH |
| PNBL-069 | Recombinant Anti-HER2 VHH Single Domain Antibody (PNBL-069) | ELISA | Llama VHH |
| HPAB-0201-FY | Recombinant llama Anti-ERBB2 Antibody (HPAB-0201-FY) | E | llama VHH |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| IAB-B008(A) | Recombinant Anti-human ERBB2 Intrabody [(D-Arg)9] | IF, FC, FuncS | scFv-(D-Arg)9 |
| IAB-B008(G) | Recombinant Anti-human ERBB2 Intrabody [+36 GFP] | WB, ICC, FuncS | scFv-(+36GFP) |
| IAB-B008(T) | Recombinant Anti-human ERBB2 Intrabody [Tat] | ICC, Neut, FuncS | scFv-Tat |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-005 | Anti-Human ErbB2 Recombinant Antibody (TAB-005) | FC, IP, ELISA, Neut, FuncS, IF, IHC | IgG1 - kappa |
| TAB-H70 | Anti-Human ERBB2 Recombinant Antibody (TAB-H70) | ELISA, IP, FC, FuncS, Neut, IF, ICC | IgG1 - kappa |
| TAB-053 | Anti-Human ERBB2 Recombinant Antibody (TAB-053) | FuncS, IF, Neut, ELISA, FC, IP, WB | IgG1 - kappa |
| TAB-033CT-S(P) | Human Anti-ERBB2 Recombinant Antibody; scFv Fragment (TAB-033CT-S(P)) | ELISA, WB | Human scFv |
| TAB-038CT-S(P) | Human Anti-ERBB2 Recombinant Antibody; scFv Fragment (TAB-038CT-S(P)) | WB | Humanized scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-761 | Anti-ERBB2 Recombinant Antibody (Margetuximab) | IF, IP, Neut, FuncS, ELISA, FC, WB | IgG1 - kappa |
| TAB-049CT | Anti-Human HER2/neu Recombinant Antibody (ChA21) | IHC, Inhib, WB, Activ, FC | Chimeric antibody (mouse/human) |
| TAB-049CT-S(P) | Anti-Human HER2/neu Recombinant Antibody scFv Fragment (ChA21) | WB | Chimeric antibody (mouse/human) |
| TAB-049CT-F(E) | Anti-Human HER2/neu Recombinant Antibody Fab Fragment (ChA21) | WB | Chimeric antibody (mouse/human) |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AGTO-G022E | Anti-ERBB2 immunotoxin 4D5 (scFv)-PE | Cytotoxicity assay, Function study | |
| AGTO-G022D | Anti-ERBB2 immunotoxin 4D5 (scFv)-DT | Cytotoxicity assay, Function study | |
| AGTO-G022G | Anti-ERBB2 immunotoxin 4D5 (scFv)-Gel | Cytotoxicity assay, Function study | |
| AGTO-G022R | Anti-ERBB2 immunotoxin 4D5 (scFv)-RTA | Cytotoxicity assay, Function study | |
| AGTO-G022S | Anti-ERBB2 immunotoxin 4D5 (scFv)-Sap | Cytotoxicity assay, Function study |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PFBL-085 | Human Anti-ERBB2 Recombinant Antibody (PFBL-085) | ELISA, WB, FuncS | Human Fab |
| PFBZ-041 | Human Anti-ERBB2 Recombinant Antibody (clone bH1); Fab Fragment | WB, IF, FuncS | Human Fab |
| HPAB-0021-YC-F(E) | Human Anti-ERBB2 Recombinant Antibody; Fab Fragment (HPAB-0021-YC-F(E)) | ELISA, Neut, FC | Human Fab |
| FAMAB-0132CQ-F(E) | Mouse Anti-ERBB2 Recombinant Antibody (clone SER4); Fab Fragment | ELISA | Mouse Fab |
| HPAB-1111LY-F(E) | Mouse Anti-ERBB2 Recombinant Antibody; Fab Fragment (HPAB-1111LY-F(E)) | ELISA | Mouse Fab |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-0191CL | Human Anti-ERBB2 Recombinant Antibody (TAB-0191CL) | ELISA, Cyt, Internalization | Human IgG |
| TAB-0192CL | Human Anti-ERBB2 Recombinant Antibody (TAB-0192CL) | ELISA, ADCC, Cyt, Internalization | Human IgG |
| TAB-0193CL | Human Anti-ERBB2 Recombinant Antibody (TAB-0193CL) | ELISA, ADCC, Cyt, Internalization | Human IgG |
| PABX-081 | Recombinant Human Anti-HER2 Antibody (Gr3) | WB, ELISA, FuncS | IgG |
| PABX-082 | Recombinant Human Anti-HER2 Antibody (Gr6) | WB, ELISA, FuncS | IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| PABC-157 | Human Anti-ERBB2 Recombinant Antibody (clone F0178C1) | Neut | Human IgG |
| HPAB-0608LY | Human Anti-ERBB2 Recombinant Antibody (HPAB-0608LY) | ELISA, Inhib, WB, FC | Humanized IgG1 |
| HPAB-0609LY | Human Anti-ERBB2 Recombinant Antibody (HPAB-0609LY) | ELISA, Inhib | Humanized IgG1 |
| HPAB-0610LY | Human Anti-ERBB2 Recombinant Antibody (HPAB-0610LY) | ELISA, Inhib | Humanized IgG1 |
| PABC-447 | Human Anti-ERBB2 Recombinant Antibody (clone 39S) | ELISA, FuncS | Human IgG1, κ |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| TAB-034CT-S(P) | Anti-Human HER2/neu Recombinant Antibody scFv Fragment (TAB-034CT-S(P)) | WB | |
| TAB-036CT-S(P) | Anti-Human HER2/neu Recombinant Antibody scFv Fragment (6B2) | ELISA, WB | |
| TAB-037CT-S(P) | Anti-Human HER2/neu Recombinant Antibody scFv Fragment (7C2/7F3) | ELISA, WB | |
| TAB-270CQ-F(E) | Mouse Anti-ERBB2 Recombinant Antibody; Fab Fragment (TAB-270CQ-F(E)) | WB, Inhib | Mouse Fab |
| PABX-082-S (P) | Recombinant Human Anti-HER2 Antibody scFv Fragment (Gr6) | WB, ELISA, FuncS | scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-024LC | Recombinant Anti-Human ERBB2 Antibody (Fab glycosylation) | ELISA | Mouse antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-145LC | Recombinant Anti-Human ERBB2 Antibody (Fc glycosylation) | ELISA | Human antibody |
| Gly-146LC | Recombinant Anti-Human ERBB2 Antibody (Fc glycosylation) | ELISA | Human antibody |
| Gly-147LC | Recombinant Anti-Human ERBB2 Antibody (Fc glycosylation) | ELISA | Human antibody |
| Gly-148LC | Recombinant Anti-Human ERBB2 Antibody (Fc glycosylation) | ELISA | Human antibody |
| Gly-149LC | Recombinant Anti-Human ERBB2 Antibody (Fc glycosylation) | ELISA | Human antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| Gly-177LC | Recombinant Anti-Human ERBB2 Antibody (Non-glycosylated) | ELISA | Humanized antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| BRD-0195MZ | Chicken Anti-ERBB2 Polyclonal IgY | WB | Chicken antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| NEUT-737CQ | Mouse Anti-ERBB2 Recombinant Antibody (clone 7.16.4) | Block, IP, IF, FC | Mouse IgG2a |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| MOR-1178 | Hi-Affi™ Rabbit Anti-ERBB2 Recombinant Antibody (clone DS1178AB) | IHC-P, IHC-Fr | Rabbit IgG |
| MOR-4586 | Hi-Affi™ Rabbit Anti-ERBB2 Recombinant Antibody (clone TH99DS) | WB | Rabbit IgG |
| MOR-4587 | Hi-Affi™ Rabbit Anti-ERBB2 Recombinant Antibody (clone TH100DS) | WB | Rabbit IgG |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| FAMAB-0132CQ-S(P) | Mouse Anti-ERBB2 Recombinant Antibody (clone SER4); scFv Fragment | ELISA | Mouse scFv |
| HPAB-1111LY-S(P) | Mouse Anti-ERBB2 Recombinant Antibody; scFv Fragment (HPAB-1111LY-S(P)) | ELISA | Mouse scFv |
| HPAB-1112LY-S(P) | Human Anti-ERBB2 Recombinant Antibody; scFv Fragment (HPAB-1112LY-S(P)) | ELISA | Human scFv |
| HPAB-M0073-YC-S(P) | Human Anti-ERBB2 Recombinant Antibody; scFv Fragment (HPAB-M0073-YC-S(P)) | ELISA, FC | Human scFv |
| HPAB-M0074-YC-S(P) | Human Anti-ERBB2 Recombinant Antibody; scFv Fragment (HPAB-M0074-YC-S(P)) | ELISA, FC | Human scFv |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| AFC-TAB-468CQ | Afuco™ Anti-ERBB2 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-468CQ) | ELISA, IHC, FC, IP, IF, FuncS | ADCC enhanced antibody |
| AFC-TAB-053 | Afuco™ Anti-ERBB2 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-053) | FuncS, IF, Neut, ELISA, FC, IP | ADCC enhanced antibody |
| AFC-TAB-761 | Afuco™ Anti-ERBB2 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-761) | IF, IP, Neut, FuncS, ELISA, FC | ADCC enhanced antibody |
| AFC-TAB-005 | Afuco™ Anti-ERBB2 ADCC Recombinant Antibody, ADCC Enhanced (AFC-TAB-005) | FC, IP, ELISA, Neut, FuncS, IF | ADCC enhanced antibody |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0424-XY91 | AbPlus™ Anti-ERBB2 Magnetic Beads (3.F2) | IP, Protein Purification | |
| VS-0724-YC1516 | AbPlus™ Anti-Erbb2 Magnetic Beads (VS-0724-YC1516) | IP, Protein Purification |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0125-FY24 | Human Anti-ERBB2 (clone 1.18.1) scFv-Fc Chimera | FC, BI | Human IgG1, scFv-Fc |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0225-XY108 | CytoStream™ Mouse Anti-ERBB2 Recombinant Antibody (clone 24D2) | FC | Mouse IgG1, kappa |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0425-YC430 | Recombinant Anti-ERBB2 Vesicular Antibody, EV Displayed (VS-0425-YC430) | ELISA, FC, Neut, Cell-uptake |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-XY2335 | Anti-ERBB2 Immunohistochemistry Kit | IHC | |
| VS-0525-XY2336 | Anti-Mouse ERBB2 Immunohistochemistry Kit | IHC |
| CAT | Product Name | Application | Type |
|---|---|---|---|
| VS-0525-YC3 | Recombinant Anti-ERBB2 Biparatopic Antibody, Symmetric scFv-Fab | ELISA | Symmetric scFv-Fab |
| VS-0525-YC6 | Recombinant Anti-ERBB2 Biparatopic Antibody, Asymmetric hetero Fab | ELISA | Asymmetric hetero Fab |
| VS-0525-YC7 | Recombinant Anti-ERBB2 Biparatopic Antibody, Asymmetric hetero HC-LC | ELISA, Inhib | Asymmetric hetero HC, common LC |
Popular Products

Application: Neut, ELISA, IF, IP, FuncS, FC, ICC

Application: IP, IF, FuncS, FC, Neut, ELISA, ICC

Application: FuncS, IF, Neut, ELISA, FC, IP, IHC

Application: FuncS, IF, Neut, ELISA, FC, IP, ICC

Application: WB, FuncS, IF, Neut, ELISA, FC, IP

Application: ELISA, FC, IP, FuncS, IF, Neut, WB

Application: IP, IF, FuncS, FC, Neut, ELISA, IHC

Application: WB, ELISA, FC, IHC, IP

Application: ELISA, IHC, FC, WB, IF, IA

Application: ELISA, Neut
For research use only. Not intended for any clinical use. No products from Creative Biolabs may be resold, modified for resale or used to manufacture commercial products without prior written approval from Creative Biolabs.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.













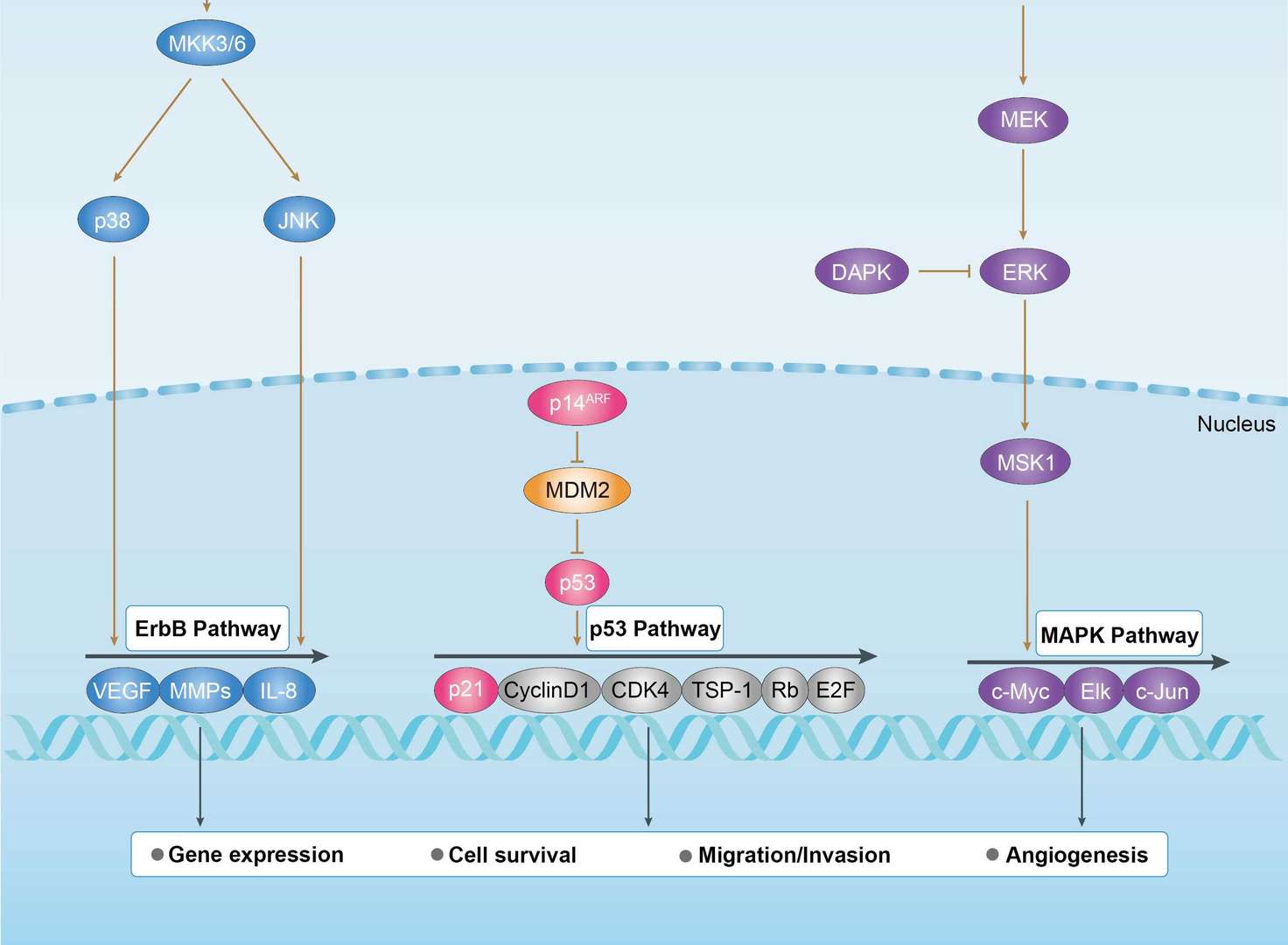 Bladder Cancer
Bladder Cancer
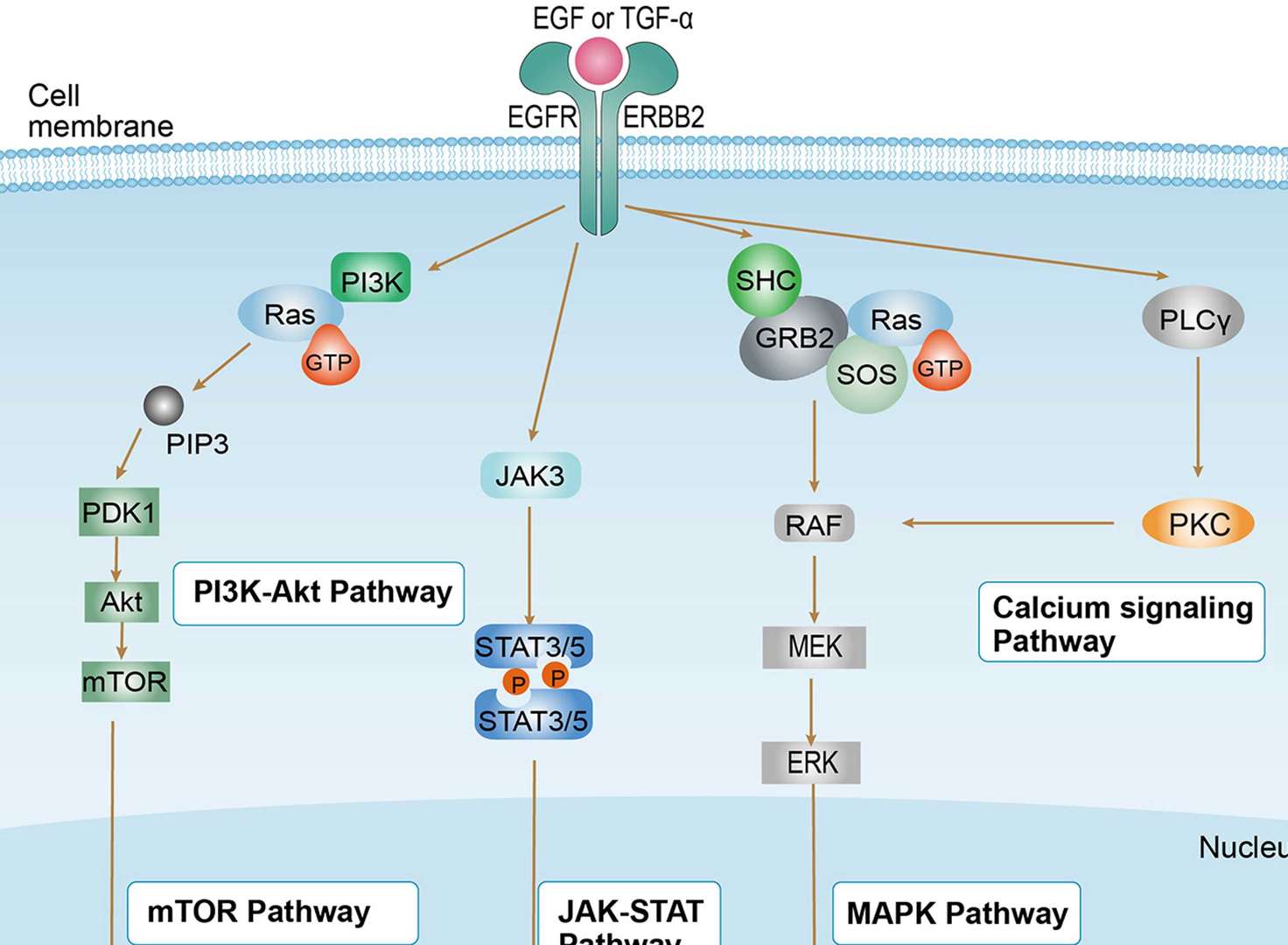 Non-small Cell Lung Cancer
Non-small Cell Lung Cancer
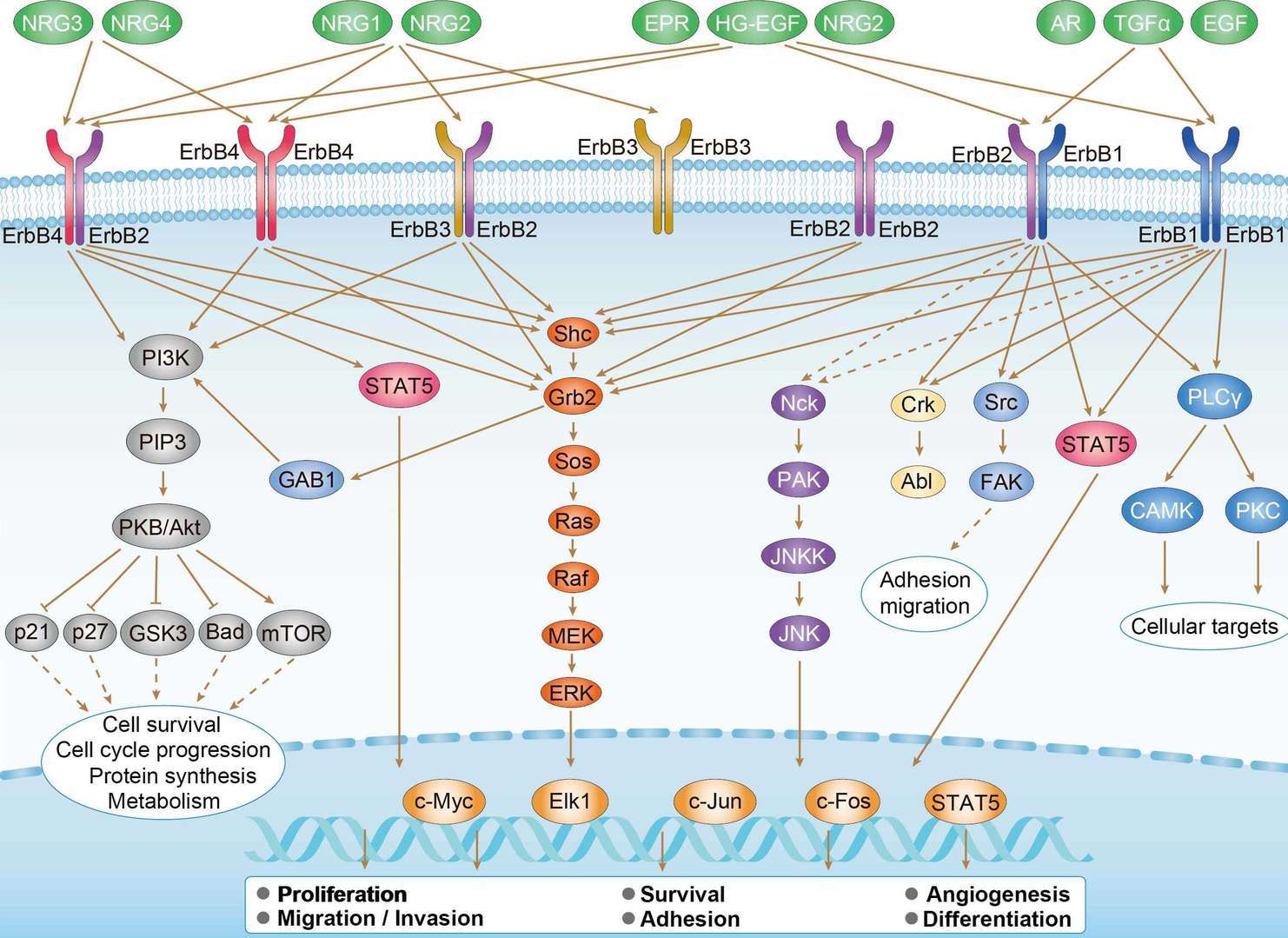 ErbB Signaling Pathway
ErbB Signaling Pathway











-2.png)
